A challenging case: Esophageal leiomyoma in a dog
A 13-year-old 35.2-lb (16-kg) neutered male bichon frise was referred to the Veterinary Emergency Clinic in Toronto, Ontario, for evaluation of a caudal thoracic mass that had been identified radiographically by the referring veterinarian.
A 13-year-old 35.2-lb (16-kg) neutered male bichon frise was referred to the Veterinary Emergency Clinic in Toronto, Ontario, for evaluation of a caudal thoracic mass that had been identified radiographically by the referring veterinarian. The dog had a history of a chronic cough, panting of four to six weeks' duration, and intermittent dyspnea. Prior medical therapy included orbifloxacin, theophylline, and prednisone. The dog's vaccination status was current, and it was receiving a monthly heartworm preventive.

Vital Stats
INITIAL PRESENTATION AND EVALUATION
On physical examination, the dog was slightly overweight. Mild dental disease was present, and mildly increased respiratory noise was noted on auscultation. The dog's temperature and heart and respiratory rates were normal. The results of a complete blood count were normal. A serum chemistry profile revealed slight hypercalcemia (3.01 mmol/L; reference range = 2.2 to 3 mmol/L). Urinalysis revealed hematuria, with 10 to 20 RBCs/HPF, which was attributed to the cystocentesis.
Thoracic radiographs obtained by the referring veterinarian revealed a 4-cm mass in the right caudodorsal thorax (Figure 1). Computed tomography (CT) showed a round 4.5-cm-diameter mass that appeared to be intraluminal in the caudal esophagus (Figure 2). The mass was well-circumscribed but slightly irregular on the right side, where it came in contact with the pulmonary parenchyma. We could not completely exclude the possibility of local invasion but considered it unlikely because the lungs appeared normal. A fine-needle aspirate was not attempted because of the mass's central location.
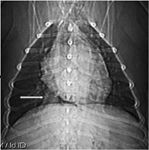
Figure 1. A ventrodorsal thoracic radiograph reveals a mass in the dog's right caudal thorax (arrow).
Our initial differential diagnoses included primary malignant esophageal neoplasia (i.e. fibrosarcoma, squamous cell carcinoma, or leiomyosarcoma), benign esophageal neoplasia (i.e. leiomyoma), esophageal granuloma, and metastatic esophageal neoplasia. We recommended esophagoscopy with possible biopsy.
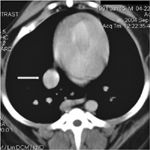
Figure 2. A transverse CT view of the dog's thorax (at the level of eighth rib) reveals a mass (arrow) just caudal to the heart and associated with the esophagus.
ENDOSCOPY AND SURGERY
Two weeks later, the dog was returned to our clinic for further evaluation with endoscopy and possible surgery. The dog was premedicated with acepromazine and hydromorphone and induced with propofol. Anesthesia was maintained with isoflurane and oxygen.
The dog was placed in left lateral recumbency, and esophagoscopy was performed with a flexible endoscope, which revealed a marked right-sided protrusion into the lumen of the distal thoracic esophagus, beyond which the endoscope was unable to pass. The mass appeared to be submucosal and had a smooth covering of normal mucosa. Given the size of the mass, we decided to perform an exploratory thoracotomy, with possible resection or biopsy.
Before surgery, meloxicam (0.2 mg/kg subcutaneously), a morphine epidural (1 ml/10 kg; 15 mg/ml), and local intercostal nerve blocks with bupivacaine (1 ml/site; 2.5 mg/ml) were administered. Cefoxitin (30 mg/kg every two hours intravenously) and intravenous fluids were given intraoperatively. Intermittent positive pressure ventilation was instituted, and a right lateral thoracotomy was performed at the eighth intercostal space.
We found a 4- to 5-cm mass in the wall of the caudal thoracic esophagus occupying about 15% to 20% of the length of the intrathoracic esophagus (Figure 3). Some consolidation of the adjacent right caudal lung was present, but no adhesions to the mass or erosion through the esophageal wall was noted. We did not resect the esophagus because we considered the risk of tension on the suture line to be excessive, given the necessary length of resection.
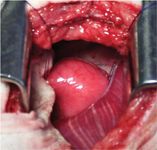
Figure 3. An intraoperative view of the esophagus after a right lateral thoracotomy shows a visible bulge in the esophageal wall.
Because the mass was well-defined on palpation and appeared submucosal on endoscopy, we attempted to dissect it. A transverse incision through the adventitial layer of the esophagus was made over the lateral aspect of the mass, which was found to be dense, encapsulated, and associated with the muscular layer of the esophagus. Enucleation of the mass was performed, with easy blunt dissection from the surrounding tissues (Figure 4), leaving the inner mucosal layer of the esophagus intact. The muscular gap and adventitia were closed in one layer with 3-0 polydioxanone in an interrupted pattern (Figure 5). A chest tube was placed before the thoracic wall was closed.
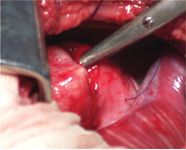
Figure 4. An intraoperative view of blunt dissection of the mass from the muscular layer with Metzenbaum scissors. Stay sutures are present in the adventitial and muscularis layer.
We repeated the esophagoscopy postoperatively, which revealed moderate bruising of the mucosa over the surgery site and complete removal of the extraluminal compression. Some redundant mucosa was present at the site, with mild swelling. Because the mucosal layer was intact, no gastrostomy tube was placed.
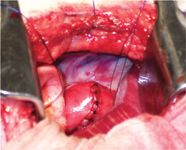
Figure 5. An intraoperative view after enucleation of the mass and closure of the esophagus.
POSTOPERATIVE CARE AND HISTOLOGIC EXAMINATION RESULTS
The dog recovered from anesthesia without complications. Intravenous fluid administration was continued for 24 hours. Postoperatively, the dog was given nothing orally for 12 hours and then fed soft food for seven days. Hydromorphone (0.05 mg/kg subcutaneously) was administered as needed for 24 hours. Meloxicam was continued for five days (0.1 mg/kg orally once a day). The chest tube was removed after 24 hours. We discharged the dog after 48 hours with instructions for the owner to restrict activity for the next four weeks and return to the referring veterinarian for a recheck and suture removal in 10 days. Further instructions included providing small, frequent meals of only soft food for seven days.
Histologic examination of the mass revealed a disorganized proliferation of mature smooth muscle cells growing in an abnormal architectural arrangement, with interlacing bundles of bland spindle cells. These findings were consistent with an esophageal leiomyoma.
FOLLOW-UP
Two weeks after surgery, reexamination by the referring veterinarian at the time of suture removal revealed that the dog showed no evidence of recurrence of respiratory signs and had a normal appetite and attitude and no evidence of dysphagia. On telephone follow-up two months after surgery, the dog was clinically normal, with no episodes of dysphagia, dyspnea, or coughing. The dog's energy level and general demeanor were greatly improved. Additional follow-up was recommended if any further clinical signs were noted.
DISCUSSION
Esophageal tumors account for less than 0.5% of all canine neoplasms.1 Most esophageal tumors occur in older animals, and they are often malignant. The most commonly reported primary esophageal tumors are sarcomas (osteosarcoma and fibrosarcoma), squamous cell carcinoma, and leiomyosarcoma; benign tumors such as leiomyoma and plasmacytoma occur less often.2 Metastatic tumors are three times more common than primary esophageal tumors and occur through local invasion from the stomach, thymus, thyroid, and heart base as well as from mammary adenocarcinoma and lymphoma.1 In dogs, the most common primary esophageal tumors are osteosarcoma and fibrosarcoma, particularly in areas where Spirocerca lupi is endemic (Africa, Asia, southeastern United States) because neoplastic transformation of S. lupi granulomas frequently occurs.3 In cats, the most common primary esophageal tumor is squamous cell carcinoma.4
Leiomyomas are benign tumors of smooth muscle origin arising in the gastrointestinal tract. The well-defined, round-to-ovoid masses are thinly encapsulated and expansile, usually with no ulceration of the overlying mucosa.5 Most (80%) arise intramurally from the muscularis propria (deep muscular layer), with a smaller percentage being pedunculated and arising from the muscularis mucosae. Esophageal leiomyomas are usually individual, slow-growing tumors, although multiple tumors can occur. In dogs, leiomyomas are most commonly found in the stomach at the gastroesophageal junction, and incidence increases with age.5 In people, leiomyomas occur most frequently in the esophagus and account for two-thirds of all benign esophageal tumors.6
Histologically, leiomyomas are distinguished from leiomyosarcomas by having a lower number of mitotic figures as well as the absence of capsular invasion and necrosis.7,8 Low-grade leiomyosarcomas may behave similarly to leiomyomas in dogs after marginal excision (removal of the entire lesion and only a small margin of surrounding tissue).8
Clinical signs
Clinical signs of esophageal tumors are most commonly due to luminal obstruction or motility dysfunction causing regurgitation and dysphagia. Other signs include weight loss, pain, and aspiration pneumonia. In some cases, respiratory signs may be the only signs.1,9 The history of dyspnea, coughing, and panting seen in this case is speculated to be due to minor episodes of aspiration secondary to dysphagia.
Esophageal tumors smaller than 2 cm seldom produce clinical signs.10 Leiomyomas at the gastroesophageal junction can be an incidental finding in asymptomatic patients at necropsy.5 However, in one dog, an esophageal leiomyoma just proximal to the lower esophageal sphincter was associated with megaesophagus and hypomotility.9 Large intra-abdominal leiomyomas and leiomyosarcomas have been reported to cause hypoglycemia in dogs as a paraneoplastic syndrome, likely because of the production of insulinlike growth factors.11 In people with esophageal leiomyomas, 15% to 50% of patients are asymptomatic, with no direct correlation between tumor size and the severity of symptoms.6
Diagnosis
Thoracic radiographs may reveal a homogeneous mass in the region of the esophagus, with gas retention and dilatation proximal to the lesion. The mass may also appear calcified.1,6,9 A barium esophagogram typically reveals a rounded or lobulated, smoothly elevated filling defect. CT can identify the tumor's size and location as well as invasiveness. Esophagoscopy reveals a submucosal tumor over which the mucosa is usually freely movable. Lumen narrowing is common, while stenosis and obstruction are rare. In people, endoscopic ultrasonography is also commonly used for diagnosis.
Definitive diagnosis is provided by histologic examination, but endoscopic biopsies are contraindicated with leiomyomas, as they increase the risk of complications and mucosal perforation during surgery.6 Endoscopic fine-needle aspiration is effective for obtaining a firm diagnosis and does not affect surgical outcome.12 Leiomyoma can be presumptively diagnosed based on endoscopic examination and CT results that indicate a well-defined, round-to-ovoid encapsulated mass, with no ulceration of the overlying mucosa or evidence of local invasion.
Treatment
Surgical resection of leiomyomas may be curative. While extensive reports are available in the human literature, reports on treatment in dogs are sparse. One study in three dogs with leiomyomas at the gastroesophageal junction treated by enucleation reported no recurrence for 8 to 18 months.13 Gastric leiomyomas have been successfully removed by both partial gastrectomy and mass dissection alone.14,15 Colorectal leiomyomas in seven dogs were treated with enucleation of the mass via the serosal or mucosal surface, with no recurrences.16
In people with esophageal leiomyomas, surgical removal is recommended if unremitting signs, a progressive increase in tumor size, or any mucosal ulceration exists.6 Conservative management and periodic endoscopy are often recommended for asymptomatic patients with tumors less than 3 cm,12 but sarcomatous transformation has been described.17
In people, standard therapy involves a lateral thoracotomy, with extramucosal enucleation of the mass. Enucleation involves separating the overlying adventitia and muscle longitudinally and performing blunt dissection, avoiding damage to the submucosa and mucosal layers.6,17 If necessary, visualization can be improved by injecting saline solution under the serosal and muscular layer to separate the planes and create a cushion between the lesion and the muscle layer.12 It is important to repair the mucosa if it is injured during dissection, as well as to close the myotomy after mass removal. Postoperative diverticula have been reported in people if the muscle layer is not reapposed.6 Tumors up to 8 cm are easily enucleated in people. Those greater than 8 cm can be removed, but the remaining muscle defect may be too large to easily close.6 If the tumor has a broad base and is tightly adherent, complete removal may not be possible by enucleation.7 Without complete removal, recurrence is likely, so debulking alone is unlikely to provide long-term relief.
Esophageal resection may be required in about 10% of people. Human mortality rates are reported from 0% to 1.3% for leiomyomas. Most patients have complete resolution of symptoms, with 89% to 94% of patients symptom-free five years later. Complications after leiomyoma enucleation in people are rare, but include diverticula, fistula, reflux esophagitis, stenosis, ulceration, and, rarely, recurrence.6 The incidence of esophagitis can increase after excision because of decreased propulsive activity and acid clearing mechanism impairment, especially in patients with a previous history of reflux. All of these complications should be considered as possible postoperative complications in dogs.
In general, esophageal surgery is prone to serious complications, including infection, regurgitation, esophagitis, dehiscence, fistula formation, stricture, and disease recurrence. The high complication rate associated with esophageal surgery has been attributed to many factors, including esophageal movement during swallowing, high wound tension, lack of a serosal layer as present in the small and large intestinal tract and omentum to help provide an early seal and promote vascularization to a repair, and the presence of a segmental blood supply to the esophagus.18,19 After esophageal surgery, particularly in cases involving resection and anastomosis or mucosal penetration or damage, use of a gastrostomy tube is recommended to decrease the amount of motion occurring at the surgical site and facilitate mucosal healing.20
In this case, esophageal resection and anastomosis were initially considered but would have required resecting 6 cm of thoracic esophagus to provide a 1-cm margin around the neoplasm. Experimentally, resection and primary anastomosis of up to 20% of the cervical esophagus and 50% of the thoracic esophagus have been reported in dogs.21 Clinically, resection of more than 3 to 5 cm increases the risk of dehiscence.20 Circumferential partial myotomy can reduce anastomotic tension and decrease the risk of dehiscence in dogs.22 Partial esophagectomy has also been reported for malignant esophageal neoplasms in dogs, with resection of the mass and then longitudinal closure of the resulting defect.3
CONCLUSION
Because tumor enucleation avoids esophageal resection and spares the mucosal surface, this technique should be considered for symptomatic esophageal leiomyomas in dogs, as expected complications would be minimal. In people, a low incidence of complications and recurrence is reported with enucleation of leiomyomas.
Stephanie A. Lister, DVM, MSc*
Veterinary Medical Teaching Hospital
College of Veterinary Medicine
Kansas State University
Manhattan, KS 66506
Kevin Isakow, BVSc, MVSc, DACVS
404 Veterinary Hospital Referral Centre
1210 Journey's End Circle
Newmarket, Ontario L3Y 8Z6
*Current address: Veterinary Referral and Critical Care
1596 Hockett Road
Manakin-Sabot, VA 23103-2226
REFERENCES
1. Ridgway RL, Suter PF. Clinical and radiographic signs in primary and metastatic esophageal neoplasms of the dog. J Am Vet Med Assoc 1979;174(7):700-704.
2. Withrow SJ. Esophageal cancer. In: Withrow SJ, Vail DM, eds. Withrow and MacEwen's small animal clinical oncology. 4th ed. St. Louis, Mo: Saunders, 2007;477-478.
3. Ranen E, Shamir MH, Shahar R, et al. Partial esophagectomy with single layer closure for treatment of esophageal sarcomas in 6 dogs. Vet Surg 2004;33(4):428-434.
4. Gualtieri M, Monzeglio MG, Di Giancamillo M. Oesophageal squamous cell carcinoma in two cats. J Small Anim Pract 1999;40(2):79-83.
5. Culbertson R, Branam JE, Rosenblatt LS. Esophageal/gastric leiomyoma in the laboratory Beagle. J Am Vet Med Assoc 1983;183(11):1168-1171.
6. Lee LS, Singhal S, Brinster CJ, et al. Current management of esophageal leiomyoma. J Am Coll Surg 2004;198(1):136-146.
7. Kim TI, Park YS, Choi EH, et al. Endoscopic resection of a large leiomyoma of the esophagus. Gastrointest Endosc 2004;59(1):129-133.
8. Farese JP, Bacon NJ, Ehrhart NP, et al. Oesophageal leiomyosarcoma in dogs: surgical management and clinical outcome of four cases. Vet Comp Oncol 2008;6(1):31-38.
9. Matros L, Jergens AE, Miles KG, et al. Megaesophagus and hypomotility associated with esophageal leiomyoma in a dog. J Am Anim Hosp Assoc 1994;30:15-19.
10. Head KW. Tumors of the alimentary tract. In: Moulten JE, ed. Tumors in domestic animals. 3rd ed. Berkely, Calif: University California Press, 1990;347-428.
11. Bagley RS, Levy JK, Malarkey, DE. Hypoglycemia associated with intra-abdominal leiomyoma and leiomyosarcoma in six dogs. J Am Vet Med Assoc 1996;208(1):69-71.
12. Wehrmann T, Martchenko K, Nakamura M, et al. Endoscopic resection of submucosal esophageal tumors: a prospective case series. Endoscopy 2004;36(9):802-807.
13. Rolfe DS, Twedt DC, Seim HB. Chronic regurgitation or vomiting caused by esophageal leiomyoma in three dogs. J Am Anim Hosp Assoc 1994;30:425-430.
14. Kerpsack SJ, Birchard SJ. Removal of leiomyomas and other noninvasive masses from the cardiac region of the canine stomach. J Am Anim Hosp Assoc 1994;30:500-504.
15. Grooters AM, Johnson SE. Canine gastric leiomyoma. Compend Contin Educ Pract Vet 1995;17:1485-1491.
16. McPherron MA, Withrow SJ, Seim HB, et al. Colorectal leiomyomas in 7 dogs. J Am Anim Hosp Assoc 1992;28:43-46.
17. Bonavina L, Segalin A, Rosati R, et al. Surgical therapy of esophageal leiomyoma. J Am Coll Surg 1995;181(3):257-262.
18. Moore TC, Goldstein J. Use of intact omentum for closure of full-thickness esophageal defects. Surgery 1959;45(6): 899-904.
19. Shamier MH, Shahar R, Johnston DE, et al. Approaches to esophageal sutures. Compend Contin Educ Pract Vet 1999;21(5): 414-421.
20. Kyles AE. Esophagus. In: Slatter D, ed. Textbook of small animal surgery. 3rd ed. Philadelphia, Pa: Saunders, 2003;573-592.
21. Saint JH, Mann FC. Experimental surgery of the esophagus. Arch Surg 1929;18:2324-2338.
22. Muangsombut J, Hankins JR, Mason GR, et al. The use of circular myotomy to facilitate resection and end-to-end anastomosis of the esophagus. J Thorac Cardiovasc Surg 1974;68(4):522-529.