A challenging case: A lethargic and depressed dog with a long history of problems
An 11-year-old 54.9-lb (27-kg) neutered male mixed-breed dog was presented to Long Island Veterinary Specialists for evaluation of lethargy and depression of several days' duration.
An 11-year-old 54.9-lb (27-kg) neutered male mixed-breed dog was presented to Long Island Veterinary Specialists for evaluation of lethargy and depression of several days' duration. Three days before presentation, the owner had observed a generalized seizure that lasted several minutes. The dog had a previous history of epilepsy, which was being treated with phenobarbital (2.5 mg/kg orally b.i.d.), and of urinary incontinence, which was being treated with phenylpropanolamine (1 mg/kg orally b.i.d.).
Pertinent history
Two and a half years before this presentation, the dog had been presented to Long Island Veterinary Specialists for evaluation of a large palpable abdominal mass that had been confirmed on abdominal ultrasonographic examination. An exploratory laparotomy had revealed a pale liver and a 30-x-20-cm splenic mass. A splenectomy had been performed. Histologic examination of a liver biopsy sample had revealed no marked lesions. Histologic examination of the splenic tissue had revealed hyperplastic nodules in the parenchyma. These lesions consisted of multiple coalescing nodules of lymphoid tissue with hyperplastic reticuloendothelial stroma interspersed throughout and focally extensive hemorrhagic and coagulation necrosis consistent with nodular splenic hyperplasia with an acute splenic hematoma. The dog had recovered from surgery without incident, and no abnormalities had been found at a recheck examination two weeks after surgery.
Two months later, the dog had been presented for evaluation of unproductive vomiting soon after a meal. Radiographic evaluation of the abdomen had been consistent with gastric dilatation-volvulus. Emergency gastric decompression had been performed with an orogastric tube. An exploratory laparotomy had confirmed gastric dilatation-volvulus; the stomach had been repositioned, and a belt-loop gastropexy had been performed. The liver had appeared mottled. Histologic examination of a liver biopsy sample had revealed vacuolar degeneration of the hepatocytes and several small discrete, nonstaining, confluent vacuoles within the cytoplasm of the hepatocytes; these changes were consistent with mild, diffuse hepatic lipidosis. The dog had recovered without incident, and no abnormalities had been found at a recheck examination three weeks after surgery.
Physical examination and diagnostic procedures
At presentation, the dog had pale mucous membranes and a distended abdomen. A complete blood count, serum chemistry profile, and urinalysis had been performed four days before presentation. Abnormal complete blood count findings (Table 1) had been a leukocytosis, a neutrophilia with a left shift, and a moderate regenerative anemia. The differential diagnoses for these abnormalities were trauma, a coagulopathy, hemorrhage, neoplasia, and immune-mediated, infectious, and inflammatory diseases. The serum chemistry profile had revealed only an elevated alkaline phosphatase activity (234 U/L, reference range = 5 to 131 U/L), which we thought could be due to drug-induced or hepatobiliary disease or an endocrinopathy. The urine had been cloudy with 1+ protein and a specific gravity of 1.040.

Table 1. Abnormal Complete Blood Count Results
We performed abdominocentesis and obtained serosanguineous fluid. Cytologic examination of the fluid revealed blood, scattered erythrophagocytic macrophages, and a few mesothelial cells, which were consistent with chronic hemoperitoneum. Our differential diagnoses for the hemoperitoneum were trauma, ruptured neoplasms, and coagulopathies. We also performed an abdominal ultrasonographic examination, which revealed two large (about 5-x-7-cm), complex masses within the right and left liver lobes. Our differential diagnoses for the masses included neoplasia and nodular hyperplasia. No abnormalities were seen on a three-view radiographic metastatic screening of the thorax.
Treatment
Because of the dog's persistent seizure activity, we discontinued the phenobarbital and initiated potassium bromide therapy at a loading dosage of 125 mg/kg given orally twice a day for five days, followed by a maintenance dosage of 20 mg/kg given orally twice a day. We performed an exploratory laparotomy the day after presentation and identified four hepatic nodules: a 4-cm nodule on the left medial lobe, a 4-cm nodule on the left lateral lobe, a 6-cm nodule on the right medial lobe, and a 1-cm nodule on the quadrate lobe. We removed the nodules on the left lateral and medial lobes with a TA 55 surgical stapler (Tyco Healthcare), the nodule on the quadrate lobe by using a ligation technique, and the entire right medial lobe with a TA 90 surgical stapler (Tyco Healthcare). We submitted all samples for histologic examination.
The dog received routine postoperative care and recovered without complication. The dog continued receiving the potassium bromide and phenylpropanolamine at the previously described dosages. The histologic examination results were consistent with multifocal ectopic splenic tissue within the liver in each sample (Figure 1). No abnormalities were found at a recheck examination two weeks after surgery.
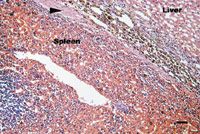
Figure 1. A photomicrograph of a section of ectopic splenic tissue in the liver from the dog in this case. Fibrous tissue (arrow) containing some hemosiderin-laden histiocytes separates the splenic tissue from hepatic tissue (hematoxylin-eosin stain; bar = 150 õm).
Follow-up
On a follow-up examination seven months after the liver lobectomy, the owner reported that the dog was more alert and active and that seizures were rare. On a routine follow-up abdominal ultrasonographic examination, we noted complex cavitary infiltrates associated with the left lateral and medial liver lobes. We obtained a fine-needle aspirate of one of the cavitary infiltrates and submitted it for cytologic examination. The smears consisted of a thick layer of blood with occasional macrophages, rare hemosiderin, and no hepatocytes. These findings were consistent with hemorrhagic fluid. No definitive diagnosis could be made. We recommended a liver biopsy, but the owner declined further testing. The owner agreed to make arrangements for follow-up examinations and abdominal ultrasonographic examinations every three or four months to monitor for changes.
One month later, the dog was presented for evaluation of lethargy and anorexia of one day's duration. The dog's packed cell volume was 22%. The owner declined diagnostic tests, and we admitted the dog for observation. Two days later, we discharged the dog because of an improved attitude and appetite and a packed cell volume of 28%. In subsequent phone conversations over several months, the owner has reported that the dog has had intermittent lethargy. Further packed cell volumes performed by the referring veterinarian have been 24% to 26%. The referring veterinarian instituted vitamin E (400 IU/day) as antioxidant therapy.
Discussion
Ectopic splenic tissue is defined as microscopically normal splenic tissue in an abnormal location.1,2 Intrahepatic splenic tissue is rarely diagnosed in people and veterinary patients. It has only been reported in four people,1,3 one pig,4 and one dog.5 In other cases, ectopic splenic tissue has been found in the pancreas, kidneys, ovaries, uterus, scrotum, abdominal wall, lungs, and pericardium.2,3,6,7
All of the reported cases of intrahepatic splenic tissue in people and in the dog occurred after splenectomy, while the pig had a normal spleen at time of diagnosis.1,3,4 In people, intrahepatic splenic tissue was found either postmortem or as an incidental finding.1,3 But in the dog, there was a history of acute lethargy and abdominal pain. The diagnosis of intrahepatic splenic tissue was made postmortem.5 Another important difference among these cases was that in three of the people, both dogs, and the pig, fibrous tissue separated the splenic tissue from the hepatic tissue1,3,4,8; in the other person,3 the hepatic and splenic tissues abutted directly. These histologic differences may reflect different causes of ectopic splenic tissue in the liver.
One cause of ectopic splenic tissue in the liver may be splenosis, which is the implantation and growth of fragments of spleen after trauma3,6,8 or elective splenectomy.8 The cells are transplanted as emboli of splenic tissue enter into the portal circulation followed by implantation and growth.6,8 In dogs, the direction of portal blood flow results in perfusion of the left liver lobes to a greater degree than the right liver lobes.5 This streamline effect results in splenic tissue perfusing the left liver lobes more than the right lobes.5 Typically, with splenosis the splenic tissue is separated from the liver by a capsule.3 The nodules are usually small but numerous with a limited blood supply.8
Another suggested possible cause of ectopic splenic tissue in the liver in this dog is that the ectopic tissue was an accessory spleen. Accessory spleens are characterized as congenital, few in number, and large. They are supplied by branches of the splenic artery.4,6,8 Microscopically, the splenic tissue is structurally the same as a normal spleen: hilus, capsule, and parenchyma. In cases of splenectomy, the ectopic tissue in the liver becomes hyperplastic once the spleen is removed.1,3,8
In this dog, distinct masses in the liver parenchyma consisted of reticuloendothelial stroma and endothelial cells that formed sinusoids. The stroma had lymphoid cells and histiocytes. Numerous hematopoietic cells were also observed. These histopathologic results were consistent with the other cases of ectopic splenic tissue,1-3,5,6 but the specific cause in all of these cases is unknown. Given this dog's history of a splenectomy with two previous liver biopsies that did not reveal splenic tissue, the fact that no branches of the splenic artery supplying the nodules were identified, and the fact that most of the nodules were in the left liver lobes, we hypothesize that the cause of this dog's intrahepatic splenic tissue was splenosis.
Magnetic resonance imaging (MRI) currently is the modality of choice to characterize hepatic lesions in people.9,10 MRI provides superior soft tissue contrast, and the documentation of characteristics of the most common lesions has made histologic confirmation unnecessary in people.9,10 To date, only one pilot study has been performed in dogs.11 Until further documentation of hepatic lesion characteristics with MRI is available, histologic confirmation is necessary, and in most cases this will involve biopsy through exploratory laparotomy.
The dog previously described in the literature and this dog presented with signs attributable to hemoperitoneum. Splenic nodules frequently develop into small hematomas as a result of marginal zone circulation failure and blood accumulation, leading to hypoxia and necrosis.12 Life-threatening hemorrhage and hemoperitoneum occur secondary to rupture of the capsule.12 Ectopic splenic tissue should be considered as a possible cause of hemoperitoneum even in dogs that have had splenectomies.
Without histologic evaluation of the splenic tissue, it is impossible to distinguish neoplastic from nonneoplastic processes. The prognosis for intrahepatic splenosis in veterinary patients is unknown. Only one dog had been reported before this case, and the dog was euthanized before the diagnosis was made. More cases would need to be reported and followed to determine the course of the disease in dogs.
Catharine A. Loughin, DVM
Dominic J. Marino, DVM, DACVS
Curtis W. Dewey, DVM, MS, DACVS, DACVIM (neurology)
Department of Surgery
Lond Island Veterinary Specialists
163 S. Service Road
Plainview, NY 11803
David A. Gamble
Antech Diagnostics
111 Marcus Ave.
Lake Success, NY 11042
REFERENCES
1. Lacerda MA, Ludwig J, Ward EM. Intrahepatic spleen presenting as a mass lesion. Am J Gastroenterol 1993;88:2116-2117.
2. al-Ahmadi M, Brundage S, Brody F, et al. Splenosis of the mesoappendix: Case report and review of the literature. J R Coll Surg Edinb 1998;43:200-202.
3. Davidson LA, Reid IN. Intrahepatic splenic tissue. J Clin Pathol 1997;50:532-533.
4. Tanimoto T, Ohtsuki Y. Heterotopic splenic tissue in the liver of a swine. J Vet Med Sci 1993;55:485-486.
5. Knostman KAB, Weisbrode SE, Marrie PA, et al. Intrahepatic splenosis in a dog.Vet Pathol 2003;40:708-710.
6. Allen J, Sands M, Baggs R. Radioisotope imaging for detection of an ectopic spleen in a macaque. J Am Vet Med Assoc 1982;181:1428-1429.
7. Bundza A, Dukes TW. Some heterotopic tissue remnants in domestic animals. Can Vet J 1978;19:322-324.
8. Yoon YS, Shin JW, Park CB, et al. Morphological structure of accessory spleen in Chinese hamsters. J Vet Sci 2000;1:73-75.
9. Semelka RC, Hussain SM, Marcos HB, et al. Perilesional enhancement of hepatic metastases: Correlation between MR imaging and histopathologic findings—Initial observations. Radiology 2000;215:84-89.
10. Semelka RC, Cance WG, Marcos HB, et al. Liver metastases: Comparison of current MR techniques and spiral CT during arterial portography for detection in 20 surgically staged cases. Radiology 1999;213:86-91.
11. Clifford CA, Pretorius ES, Weisse C, et al. Magnetic resonance imaging of focal splenic and hepatic lesions in the dog. J Vet Intern Med 2004;18:330-338.
12. Marino DJ. Diseases of the spleen. In: Kirk's current veterinary therapy XIII. Philadelphia, Pa: WB Saunders, 2000;520-524.