A challenging case: Progressive, generalized pain in a young English bulldog
A 1-year-old 58.3-lb (26.5-kg) castrated male English bulldog presented to the Mathew J. Ryan Veterinary Hospital at the University of Pennsylvania for evaluation of progressive pain that was difficult to localize.
A 1-year-old 58.3-lb (26.5-kg) castrated male English bulldog presented to the Mathew J. Ryan Veterinary Hospital at the University of Pennsylvania for evaluation of progressive pain that was difficult to localize. The dog's vaccination status was current for rabies, canine distemper, and parvovirus. The dog had not traveled outside of Pennsylvania, and there was no history of trauma.

Vital Stats
Ten days before admission to the University of Pennsylvania, the dog had been evaluated by a local veterinarian for continuous crying out, shaking, and panting. The complete blood count, serum chemistry profile, and urinalysis results had been normal. Empiric treatment had been initiated with prednisolone acetate (1 mg/kg subcutaneously once), diazepam (0.25 mg/kg orally every 8 hours), and carprofen (2.2 mg/kg orally every 12 hours). Over the next 10 days, the dog had progressively exhibited more pain, had been unable to lower its head and neck to eat, and had developed progressive pelvic limb lameness. Consequently, the dog was reevaluated by the local veterinarian, given another injection of prednisolone (1 mg/kg subcutaneously), and referred to the University of Pennsylvania.
PHYSICAL AND NEUROLOGIC EXAMINATIONS
On admission to the University of Pennsylvania, physical examination revealed mild upper airway stridor. The dog had normal heart and respiratory rates and a normal rectal temperature. The dog's sensorium was quiet and responsive. The patient had a normal gait in its thoracic limbs but a stiff and short-strided gait in both pelvic limbs. The dog had difficulty rising from a recumbent position and was reluctant to walk and unwilling or unable to run.
Postural reactions were normal in all limbs. During the postural reaction tests, the dog would cry out. Muscle tone was normal in all limbs. No muscle atrophy was detected. The dog's spinal reflexes and the results of a cranial nerve examination were normal. The dog appeared to be in extreme pain when the spinous processes were palpated and when the vertebral column was manipulated, especially the caudal lumbar vertebrae and sacrum.
Given the patient's abnormal pelvic gait and sensitivity to palpation in the lumbar region, we presumed that the lesion's neuroanatomic location was between L4 and S2. Conditions that may cause this presentation of generalized pain or pain that is difficult to localize to a specific area include degenerative, neoplastic, infectious, inflammatory, immune-mediated, or traumatic diseases that affect the intervertebral disks, facet joint capsules, dorsal root ganglia and sensory nerves, vertebral ligaments, musculature, periosteum, or meninges.1
DIAGNOSTIC TESTING AND DIFFERENTIAL DIAGNOSES
The results of a serum chemistry profile, complete blood count, and urinalysis were within reference ranges. Serologic testing for neutralizing antibodies to canine distemper virus revealed a titer of 1:192, which was likely a result of the previous vaccinations for canine distemper virus. In addition, the serum concentrations of IgG directed against Neospora caninum and IgM and IgG directed against Toxoplasma gondii were normal. A serum latex agglutination test for Cryptococcus neoformans was negative. Serum creatine kinase activity was normal. Radiographic examination of the thorax revealed no abnormalities.
The dog was anesthetized, and survey radiographs of the vertebral column revealed widening and lucency of the caudal physis of L5 with loss of the normal physeal margins and a suspected pathologic fracture. Scoliosis of the thoracic vertebral column, most likely a result of congenital vertebral abnormalities from T5 to T11, was evident. Myelographic evaluation was performed by injecting 0.4 ml/kg iohexol into the cerebellomedullary cistern and revealed no static or dynamic compression of the spinal cord at the level of the physeal fracture (Figure 1).

1. A lateral vertebral radiograph of the lumbar region acquired during myelography of the dog in this case. Note the widening and lucency of the caudal physis of L5 with loss of normal physeal margins and pathologic fracture of the caudal end plate. The caudal L4 vertebral body and the L5 vertebral body have increased opacity. There is no evidence of static or dynamic compression of the spinal cord at the level of the physeal fracture.
Cerebrospinal fluid was collected from the cerebellomedullary cistern at the time of the myelogram. Analysis of the fluid revealed a total protein concentration of 24 mg/dl (normal ≤ 25 mg/dl) and a nucleated cell count of 6 cells/μl (normal ≤ 5 nucleated cells/μl) consisting of small lymphocytes and nonphagocytic macrophages. Aerobic bacterial culture of the cerebrospinal fluid yielded no growth.
The patient's physical examination and radiographic findings were consistent with vertebral physitis. However, we could not rule out a traumatic fracture or neoplastic or metabolic process affecting the physis. The owner declined additional diagnostic tests such as bacterial cultures of the blood and urine and fluoroscopic-guided aspiration of the vertebra.
TREATMENT AND FOLLOW-UP
We suspected a bacterial cause of the vertebral physitis and initiated treatment with cephalexin (22 mg/kg orally every 8 hours for three weeks). Analgesia was provided by transdermal administration of fentanyl (2.8 μg/kg). After three days of therapy, the dog developed mucopurulent nasal discharge and began sneezing. Enrofloxacin (5 mg/kg orally b.i.d. for 20 days) and metronidazole (22 mg/kg orally b.i.d. for seven days) were added to treat a presumptive upper respiratory tract infection and in an attempt to prevent pneumonia. The fentanyl patch was removed, and the patient was discharged with instructions to the owner to firmly restrict exercise at home.
On reevaluation seven days later, the patient had mild mucopurulent nasal discharge. Pain was not evident on palpation of the vertebral column, but the dog's gait remained stiff and short-strided in the pelvic limbs. Three weeks after the initial examination, the antibiotic therapy was discontinued.
Four weeks after the initial examination, the dog was reevaluated for recurrent crying, panting, and shaking. The patient was in extreme pain upon palpation of the lumbar vertebrae; however, the gait remained unchanged from previous examinations. Complete blood count and serum chemistry profile results were within reference ranges. Cystocentesis was performed, and a urinalysis revealed a moderate amount of a mixed population of bacteria that were predominantly bacilli. Aerobic bacterial culture of the urine grew > 105 colonies/ml of Escherichia coli and > 105 colonies/ml of Enterococcus species. An aerobic bacterial culture of the blood yielded no growth. The results of immunofluorescent antibody testing for Brucella canis were negative.
The dog was anesthetized, and vertebral radiographs were obtained of the previously affected site. Degenerative changes of the caudal physeal region of L5 and bony callus formation ventral and lateral to the pathologic fracture were evident (Figure 2). We reinstituted antibiotic therapy consisting of cephalexin (22 mg/kg orally every 8 hours) and enrofloxacin (5 mg/kg orally every 12 hours). Forty-eight hours later, the patient's gait and level of pain improved dramatically, and the patient was discharged with physical activity restriction instructions.

2. A lateral vertebral radiograph taken on Day 28 revealed degenerative changes of the caudal physeal region of L5 and bony callus formation ventral to the physeal fracture.
Twenty days later (Day 48), the patient did not show signs of pain upon palpation of the vertebral column; its gait remained unchanged from previous examination findings. Radiographs of the lumbar region were consistent with further remodeling of the caudal physeal region of L5 (Figure 3). We continued the cephalexin and enrofloxacin for an additional 12 weeks. During reevaluation on Days 90 and 138, the dog did not show evidence of pain, but the stiff, short-strided pelvic limb gait remained.

3. A lateral vertebral radiograph taken on Day 48. Note the bony callus and ventral ossification of L5 indicating further remodeling of the previous physitis site.
DISCUSSION
This case describes a dog that had poorly localized pain. Conditions that result in generalized pain or pain that is difficult to localize to a specific area include diseases that affect the intervertebral disks, facet joint capsules, dorsal root ganglia and sensory nerves, vertebral ligaments, musculature, periosteum, or meninges.1 Because of the diagnostic test results as well as the patient's response to therapy, it was ultimately determined that the dog had vertebral physitis.
Types of vertebral infections
Vertebral infections are classified based on the portion of the vertebra affected or the surrounding structures affected. Discospondylitis is an infection of the intervertebral disk and adjacent vertebral end plates, resulting in symmetrical lysis of the vertebral end plates with reactive sclerosis in the vertebral bodies.2-4 Vertebral physitis is inflammation, lysis, and reactive bone involving the caudal physeal zone of affected vertebrae without involvement of the intervertebral disk.2-4 Vertebral osteomyelitis or spondylitis is an infection confined to the vertebral body.4
Vertebral physitis occurs primarily in dogs under 2 years of age.2 In contrast, discospondylitis occurs most commonly in middle-aged to older dogs.2 In cases of vertebral physitis, the lumbar vertebrae are most frequently affected.2 In cases of discospondylitis, common areas include the thoracic and lumbar vertebral column and the lumbosacral disk space, but any intervertebral disk space can be affected.4-6 Vertebral osteomyelitis is seen more commonly in horses and ruminants than in small animals7-11 ; it can occur at any vertebra. In ruminants, vertebral osteomyelitis has been diagnosed concurrently with an adjacent spinal abscess known as epidural empyema. Epidural empyema has been reported in ruminants as well as dogs.10-15
Causes
Migrating plant awns (grass seeds, foxtails) have been described as a cause of discospondylitis and vertebral osteomyelitis in dogs in certain regions in the United States. Other causes of vertebral infections may include penetrating wounds, extension of an infection adjacent to the vertebrae, epidural anesthesia, or previous disk or vertebral surgery. Many cases of vertebral infection do not have an apparent source of infection.4,5
Most vertebral infections are thought to develop through hematogenous spread of bacteria or fungi from a primary infection involving other areas of the body such as the urogenital tract, skin, heart valves, and oral cavity.2,4,5 Urinary tract infections are often associated with vertebral infections, but a direct effect is rarely proved.4 However, similarities exist between the common age at which dogs are affected by vertebral infections and urinary tract infections. Dogs under the age of 3 years were shown to be at an increased risk for recurrent or persistent urinary tract infections, while dogs older than 10 years of age were at a decreased risk for recurrent urinary tract infections.16 Urogenital infections may be more common in young animals, and, although there may not be a direct correlation, dogs that have vertebral infections are more commonly younger or middle-aged.2 Urinary tract infections may not be associated with vertebral infections since urinary tract infections occur more frequently in female dogs than in males, whereas discospondylitis is more common in males.17,18
Pathophysiology and vertebral blood flow
In order to understand the pathophysiology involved in vertebral infections, knowledge of the vascular anatomy and blood flow of the vertebral column is needed. The vertebral venous system is a set of valveless vessels that carries blood under low pressures. It connects with and provides bypasses for the portal, pulmonary, and caval systems of veins and can provide a pathway for disease to spread among organs.19,20
The internal ventral vertebral venous plexus is made up of paired longitudinal valveless vessels that extend from the caudal vertebrae to the foramen magnum and course along the floor of the vertebral canal ventral to the spinal cord. Blood may flow cranially or caudally depending on pressure.19,21,22 The internal ventral vertebral venous plexus drains into the vertebral bodies where it joins the basivertebral veins. Venous blood exits the vertebral bodies via the basivertebral veins. The basivertebral veins join the internal ventral vertebral plexus with the external ventral vertebral plexus. In the lumbar region, the basivertebral veins are largest, and in this region they connect with lumbar veins by the ventral venous plexus.22 This arrangement may explain the frequent involvement of the lumbar region in vertebral physitis. The intervertebral veins anastomose the vertebral plexuses and extravertebral veins. These vessels drain the vertebral regions and are closely associated with surrounding organs.22 Hematogenous extension of infection or neoplasia occurs through connections with surrounding tissues.19-21,23 Retrograde flow in the vertebral veins may contribute to localization of infection or neoplasia in the vertebrae.5
Metastasis of malignancies involving the prostate, bladder, or uterus to the lumbar spine has been seen in human and veterinary medicine.24-31 In addition, infections such as pyelonephritis, perinephritis, prostatitis, and cystitis have preceded vertebral infections.24,26,32 In the 1940s, it was hypothesized that such patterns of metastasis and spread of infection were caused by an increase in intra-abdominal pressure that prevented blood flow from the pelvis to the inferior vena cava, causing shunting of the blood to the vertebral vein system.19,21 In the normal course of blood flow, radiopaque material injected into the dorsal vein of the penis of monkeys coursed to the inferior vena cava. However, with increased intra-abdominal pressure, the injected material ascended to the internal vertebral venous plexus rather than the vena cava.19,21
In rats and rabbits, tumor cells introduced into the femoral vein while intra-abdominal pressure was elevated bypassed the lungs and led to tumor growth in the lumbar vertebrae.33 Increased intra-abdominal pressure may occur in animals from activity such as coughing, sneezing, or straining to defecate.19
In vertebral physitis, the infection is thought to involve the highly vascular metaphyseal and epiphyseal capillary beds.2,34 Blood in this area flows relatively slowly, and the primary spongiosa lacks white blood cells, allowing bacterial colonization.35 In discospondylitis, the infection is thought to develop from the same epiphyseal capillaries with rapid diffusion of microorganisms into the avascular intervertebral disk and the adjacent vertebral end plates.2,4,35
Clinical and radiographic signs
Clinical signs of vertebral infections may include pain, fever, anorexia, lethargy, and neurologic deficits such as ataxia and paresis. The neurologic signs depend on the location and severity of the lesion.2,5 The most common clinical signs of vertebral physitis are spinal hyperesthesia, lethargy, and pelvic limb weakness.2
Early radiographic signs of vertebral physitis include widening and lucency of the caudal physis with loss of normal physeal margins. Later radiographic findings include a collapsed or narrowed physis surrounded by sclerosis and remodeling of the ventrocaudal region of the vertebra. Vertebral end plates are normal, and no changes are noted at the intervertebral disk space. These features distinguish vertebral physitis from discospondylitis.2,4
In cases of discospondylitis, changes in the intervertebral disk space are observed, and the vertebral end plates exhibit symmetrical lysis and the vertebral bodies exhibit sclerosis.2,4 There is generally about a two- to four-week lag time in the onset of clinical signs of discospondylitis and the first radiographic evidence.6,36 In addition, radiographic changes during recovery lag behind clinical improvement. A study of radiographic changes during recovery from discospondylitis showed that in older dogs, this lag period may be three to nine weeks, while it is shorter in dogs under 1 year of age.36 Since vertebral physitis is a disease similar to discospondylitis, these lag times in radiographic changes should be taken into consideration when interpreting initial and follow-up radiographs in dogs with vertebral physitis.
Other imaging study findings
Other diagnostic imaging studies can be used in evaluating vertebral infections. Myelography may show evidence of extradural compression, such as attenuation of the contrast medium column in the subarachnoid space over the affected disk space or vertebra. Computed tomography can identify bone pathology and paravertebral soft tissue swelling.4,37 Magnetic resonance imaging (MRI) is the diagnostic procedure of choice to identify infected vertebrae, especially in the early stages of the disease process.4,32,37

4. A sagittal midline T2-weighted magnetic resonance image of the cervical region from an 11-year-old Weimaraner with intervertebral disk disease. The degenerative disks are characterized by hypointensity of the nucleus pulposus (arrows).
MRI should be considered in cases in which radiographic imaging or computed tomography does not reveal distinct evidence of a vertebral infection. With MRI, degenerative changes of the intervertebral disk are characterized by a loss of signal intensity of the nucleus pulposus on T2-weighted images (Figure 4), while infection causes T2-weighted hyperintensity of both the nucleus pulposus and the anulus fibrosus (Figure 5).37
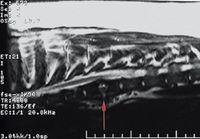
5. A sagittal midline T2-weighted magnetic resonance image of a 1.5-year-old greater Swiss mountain dog. There is an irregularly marginated area of increased signal intensity centered at an intervertebral disk space in the midthoracic spine (arrow). At this site, the normal smooth, linear, low signal pattern of the vertebral end plates has been lost, consistent with a destructive process expanding outward from the intervertebral disk space. The adjacent bone of the vertebral bodies is expanded in dorsoventral dimension and reduced in signal, consistent with remodeling and sclerosis. Reactive, irregular periosteal new bone is visible bridging the ventral aspect of the disk space. These changes are consistent with chronic active infectious discospondylitis.
Fluoroscopic-guided percutaneous needle aspiration with cytologic examination and bacterial and fungal cultures are indicated in dogs that do not respond to initial antibiotic therapy or if the diagnosis is unclear.4
Microorganisms involved
The most frequently isolated microorganisms in discospondylitis are coagulase-positive Staphylococcus species (Staphylococcus aureus, Staphylococcus intermedius).38 Brucella canis is another possible cause of discospondylitis, likely from hematogenous spread from a genital infection.6 Fungal infections are less common, with Aspergillus species being the most frequent fungal cause of discospondylitis. German shepherds are overrepresented in cases of disseminated aspergillosis.39,40
Overall, physitis is less common than discospondylitis and, thus, information in the literature is limited. Escherichia coli, Serratia species, and Streptococcus species were identified in the urine in three dogs with vertebral physitis.2 One dog with concurrent vertebral physitis and discospondylitis was seropositive for Brucella canis infection. Three other dogs with vertebral physitis revealed two positive biopsy culture samples for Actinobacter species and Enterococci species.2 Bacterial culture of the blood in one dog with vertebral physitis revealed Staphylococcus intermedius.41
Treatment
Treatment of vertebral physitis consists of exercise restriction, long-term antibiotic therapy, and analgesics. Bacterial and fungal cultures of urine, blood, or infected tissue are indicated to establish a definitive diagnosis and to aid in the choice of appropriate antibiotic or antifungal treatment.3,5 Additionally, Brucella canis serology should always be performed if a vertebral infection is identified or suspected because of the zoonotic potential and guarded prognosis.6 Since vertebral physitis and discospondylitis are both vertebral infections thought to arise from an infection elsewhere in the body, it may be assumed that the same organisms identified with discospondylitis may also cause vertebral physitis. Thus, in patients with discospondylitis or physitis, empirical therapy with an antibiotic effective against coagulase-positive Staphylococcus species such as a first-generation cephalosporin or a beta-
lactamase-resistant penicillin is a logical choice while awaiting culture results or when aerobic cultures fail to identify a causative bacterial agent.38 Treat dogs with neurologic deficits or severe pain more aggressively with broad-spectrum antibiotics such as a combination of a beta-lactam antibiotic as well as an aminoglycoside or fluoroquinolone.18
Reassess patients that fail to improve within five days of starting antibiotic treatment. Continue antibiotic treatment for at least eight weeks to treat the infection and prevent relapse.4 In a retrospective study on the clinical features of discospondylitis, antibiotic therapy was continued for a median of 53 weeks and, because of the study results, the authors recommended treatment with antibiotic therapy until complete resolution of radiographic evidence of active disease.18
SUMMARY
Vertebral physitis is a vertebral infection that should be suspected in cases of lameness and back pain in young dogs. Bacterial cultures of urine and blood with sensitivity testing plus serologic testing for Brucella canis should be performed in all dogs suspected of having a vertebral infection.4 Vertebral physitis is diagnosed based on clinical signs; the results of radiography, computed tomography, or MRI; bacteriologic studies; and response to antibiotic treatment.
ACKNOWLEDGMENTS
The authors thank Dr. Sheldon Steinberg and Dr. Charles Vite for their collective expertise and clinical input with this patient, Dr. H. Daniel Cantwell for his interpretation of the radiograph in Figure 1, and Dr. Justin Goggin for his interpretation of the MRI in Figure 5.
Laura C. Tepper, DVM
Department of Clinical Sciences
College of Veterinary Medicine
Mississippi State University
Mississippi State, MS 39762-6100
Eric N. Glass, MS, DVM, DACVIM (neurology)
Red Bank Veterinary Hospital
197 Hance Ave.
Tinton Falls, NJ 07724
Marc Kent, DVM, DACVIM (neurology and internal medicine)
Department of Small Animal Medicine and Surgery
College of Veterinary Medicine
University of Georgia
Athens, GA 30602-7390
REFERENCES
1. Webb AA. Potential sources of neck and back pain in clinical conditions of dogs and cats: a review. Vet J 2003;165:193-213.
2. Jimenez MM, O'Callaghan MW. Vertebral physitis: a radiographic diagnosis to be separated from discospondylitis: A preliminary report. Vet Radiol Ultrasound 1995;36:188-195.
3. Lame EL. Vertebral osteomyelitis following operation on the urinary tract or sigmoid; the third lesion of an uncommon syndrome. Am J Roentgenol Radium Ther Nucl Med 1956;75:938-952.
4. Thomas WB. Diskospondylitis and other vertebral infections. Vet Clin North Am Small Anim Pract 2000;30:169-182.
5. LeCouteur RA, Grandy JL. Diseases of the spinal cord. In: Ettinger SJ, Feldman EC, eds. Textbook of small animal internal medicine. 6th ed. Philadelphia, Pa: WB Saunders Co, 2005;858-861.
6. Oliver JE, Lorenz MD, Kornegay JN. Pelvic limb paresis, paralysis, or ataxia. In: Oliver JE, Lorenz MD, Kornegay JN, eds. Handbook of veterinary neurology. 3rd ed. Philadelphia, Pa: WB Saunders Co, 1997;155-157.
7. Hudson NPH, Mayhew IG. Radiographic and myelographic assessment of the equine cervical vertebral column and spinal cord. Equine Vet Educ 2005;17:34-38.
8. Healy AM, Doherty ML, Monaghan ML, et al. Cervico-thoracic vertebral osteomyelitis in 14 calves. Vet J 1997;154:227-232.
9. Giguere S, Lavoie JP. Rhodococcus equi vertebral osteomyelitis in 3 quarter horse colts. Equine Vet J 1994;26:74-77.
10. Divers TJ. Acquired spinal cord and peripheral nerve disease. Vet Clin North Am Food Anim Pract 2004;20:231-242.
11. Mayhew IG. Tetraparesis, paraparesis and ataxia of the limbs and episodic weakness. In: Mayhew IG, ed. Large animal neurology: a handbook for veterinary clinicians. Philadelphia, Pa: Lea & Febiger, 1989;277-279.
12. Lavely JA, Vernau KM, Vernau W, et al. Spinal epidural empyema in seven dogs. Vet Surg 2006;35:176-185.
13. Naughton JF, Tucker RL, Bagley RS. Radiographic diagnosis—paraspinal abscess in a dog. Vet Radiol Ultrasound 2005;46:23-26.
14. Braun U, Schweizer G, Gerspach C, et al. Clinical findings in 11 cattle with abscesses in the thoracic vertebrae. Vet Rec 2003;152:782-784.
15. Jerram RM, Dewey CW. Suspected spinal epidural empyema and associated vertebral osteomyelitis (physitis) in a dog. J Vet Emerg Crit Care 1998;8:103-108.
16. Seguin MA, Vaden SL, Altier C, et al. Persistent urinary tract infections and reinfections in 100 dogs (1989-1999). J Vet Intern Med 2003;17:622-631.
17. Stiffler KS, Stevenson MA, Sanchez S, et al. Prevalence and characterization of urinary tract infections in dogs with surgically treated type 1 thoracolumbar intervertebral disc extrusion. Vet Surg 2006;35:330-336.
18. Burkert BA, Kerwin SC, Hosgood GL, et al. Signalment and clinical features of diskospondylitis in dogs: 513 cases (1980-2001). J Am Vet Med Assoc 2005;227:268-275.
19. Batson OV. The role of the vertebral veins in metastatic processes. Ann Intern Med 1942;16:38-45.
20. Worthman RP. The longitudinal vertebral venous sinuses of the dog. II. Functional aspects. Am J Vet Res 1956;17:349-363.
21. Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg 1940;112:138-149.
22. Evans HE. Veins. In: Evans HE, ed. Miller's anatomy of the dog. 3rd ed. Philadelphia, Pa: WB Saunders Co, 1993;713-715.
23. Reinhard KR, Miller ME, Evans HE. The craniovertebral veins and sinuses of the dog. Am J Anat 1962;111:67-87.
24. Deming CL, Zaff F. Metastatic vertebral osteomyelitis complicating prostatic surgery. Trans Am Assoc Genitourin Surg 1943;35:287-305.
25. Hentschel SJ, Mendel E, Singh S, et al. Metastatic prostate carcinoma to the intradural extramedullary spinal compartment. Case report. J Neurosurg 2004;100:375-377.
26. Kutzler MA, Yeager A. Prostatic diseases. In: Ettinger SJ, Feldman EC, eds. Textbook of small animal internal medicine. 6th ed. Philadelphia, Pa: WB Saunders Co, 2005;1812-1817.
27. Tamada T, Sone T, Jo Y, et al. Three-dimensional trabecular bone architecture of the lumbar spine in bone metastasis from prostate cancer: comparison with degenerative sclerosis. Skeletal Radiol 2005;34:149-155.
28. Perry RE, Weller RE, Dagle GE, et al. Transitional cell carcinoma of the bladder with skeletal metastases in a dog. J Am Anim Hosp Assoc 1989;25:547-551.
29. Mellanby RJ, Chantrey JC, Baines EA, et al. Urethral haemangiosarcoma in a boxer. J Small Anim Pract 2004;45:154-156.
30. Oyanagi K, Ogata K, Takeda S, et al. Widespread vertebral and epidural venous plexus metastasis of prostatic carcinoma presenting wedge-shaped radial lesions in the spinal cord. Neuropathology 2003;23:296-300.
31. Nishijima Y, Uchida K, Koiso K, et al. Clinical significance of the vertebral vein in prostate cancer metastasis. Adv Exp Med Biol 1992;324:93-100.
32. Kraft SL, Mussman JM, Smith T, et al. Magnetic resonance imaging of presumptive lumbosacral discospondylitis in a dog. Vet Radiol Ultrasound 1998;39:9-13.
33. Coman DR, deLong RP. The role of the vertebral venous system in the metastasis of cancer to the spinal column; experiments with tumor-cell suspensions in rats and rabbits. Cancer 1951;4:610-618.
34. Wood GW. Infections of the spine. In: Canale ST, ed. Campbell's operative orthopaedics. 9th ed. St. Louis, Mo: Mosby, 1998;3093.
35. Gilson SD, Scheartz PD. Acute hematogenous osteomyelitis in a dog. J Am Anim Hosp Assoc 1989;25:684-688.
36. Shamir MH, Tavor N, Aizenberg T. Radiographic findings during recovery from discospondylitis. Vet Radiol Ultrasound 2001;42:496-503.
37. Gonzalo-Orden JM, Altónaga JR, Orden MA, et al. Magnetic resonance, computed tomographic and radiologic findings in a dog with discospondylitis. Vet Radiol Ultrasound 2000;41:142-144.
38. Dewey CW. Disorders of the cauda equina. In: Dewey CW, ed. A practical guide to canine and feline neurology. 1st ed. Ames: Iowa State Press, 2003;348-351.
39. Berry WL, Leisewitz AL. Multifocal Aspergillus terreus discospondylitis in two German shepherd dogs. J S Afr Vet Assoc 1996;67:222-228.
40. Harkin KR. Aspergillosis: an overview in dogs and cats. Vet Med 2003;98:602-618.
41. Walker MC, Platt SR, Graham JP, et al. Vertebral physitis with epiphyseal sequestration and a portosystemic shunt in a Pekingese dog. J Small Anim Pract 1999;40:525-528.