Clinical Exposures: A dog with a nonhealing flank wound
A 4.5-year-old intact female Labrador retriever was presented to The Ohio State University Teaching Hospital for evaluation of a nonhealing wound on the left caudal flank.
A 4.5-year-old intact female Labrador retriever was presented to The Ohio State University Veterinary Teaching Hospital (OSU) for evaluation of a nonhealing wound on the left caudal flank. The farm dog spent about 90% of its time outdoors.
History
A left flank abscess had initially been noted by the owner and diagnosed by the referring veterinarian about two years before presentation to OSU. Treatment by the referring veterinarian had included débridement of the abscess and antimicrobial therapy (cephalexin, orbifloxacin, and ciprofloxacin used in combination or individually during various treatment episodes) based on the results of bacterial culture and antimicrobial sensitivity testing of representative tissue samples that had also been submitted for histologic evaluation.
Local surgical exploration and débridement had not revealed a nidus of infection. Histology had revealed chronic, focally extensive necrotizing pyogranulomatous dermatitis with sinus tracts, cavitations, and fibrosis. Aerobic bacterial culture results had revealed Pseudomonas aeruginosa and Streptococcus uberis. The swelling and drainage would diminish with the antimicrobial therapy, but these signs would return shortly after the antibiotic course ended.
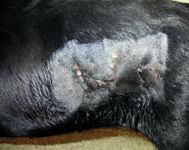
Figure 1. The cutaneous draining tracts in the dog's left flank on presentation.
Physical examination and diagnostic testing
On initial physical examination at OSU, the dog had no abnormalities other than a series of draining tracts in its left flank with a small amount of caseous exudate (Figure 1). The dog weighed 80.2 lb (36.4 kg), with a body condition score between 2 and 3 (out of 5). The results of the complete blood count (CBC) and serum chemistry profile were unremarkable except for an increased globulin concentration (3.6 g/dl; reference range = 2.2 to 2.9 g/dl).
Differential diagnoses for an increased globulin concentration include chronic antigenic stimulation secondary to infectious or inflammatory disease, immune-mediated disorders, neoplasia, and idiopathic causes (macroglobulinemia and benign monoclonal gammopathy). Because the globulin concentration was only mildly elevated and infection or inflammation was suspected to be the cause, protein electrophoresis and other diagnostics were not pursued to rule out other differential diagnoses of hyperglobulinemia.
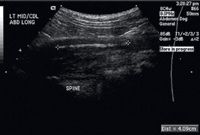
Figure 2. A longitudinal ultrasonogram of the foreign body and associated draining tract. The hyperechoic linear foreign body is between the + signs. The aorta is not seen in this image.
Cytologic evaluation of the fluid draining from the wound revealed a septic exudate. Most of the cells were degenerate neutrophils with multiple types of intracellular and extracellular bacterial rods and cocci. No other etiologic agents were found.
The results of an abdominal radiographic examination were normal, but because of the location of the skin lesion, abdominal ultrasonography was performed to rule out a retroperitoneal abscess (Figures 2 & 3). Intra-abdominal structures were normal. However, a 4-cm long, linear hyperechoic structure was seen in the left midlumbar hypaxial musculature, running parallel to the vertebral column and within 1 cm of the aorta. The soft tissues surrounding the foreign body were hypoechoic. A 7-cm diameter hypoechoic linear tract extended from the hyperechoic structure to the draining skin lesions of the left flank. The final ultrasonographic diagnosis was a retroperitoneal linear foreign body within the hypaxial musculature.
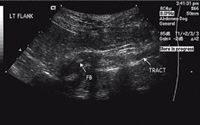
Figure 3. A transverse ultrasonogram of the foreign body (FB) and associated draining tract.
Treatment and follow-up
Because of the deep retroperitoneal location of the foreign body and its proximity to the aorta, an exploratory celiotomy was performed. The foreign body's location was not evident on gross inspection of the abdomen during initial exploration. Intraoperative ultrasonography was used to localize the foreign body within the hypaxial musculature. An incision was made into the retroperitoneal space, and the ureter and aorta were preserved. A 3-cm incision was made in the musculature over the suspected foreign body, and a 6-cm stick was removed intact (Figures 4 & 5). Samples of the tissue surrounding the foreign body were submitted for fungal and bacterial culture.

Figure 4. An intraoperative photograph of the foreign body deep in the retroperitoneal space in the hypaxial musculature.
The retroperitoneum and abdominal cavity were lavaged copiously with saline solution. No attempt was made to remove the draining tracts. Intravenous enrofloxacin (one dose; 21 mg/kg) was administered. Omentum was placed over the foreign body site and tacked into place by using simple interrupted sutures using 4-0 poliglecaprone 25 suture. The linea alba was closed in a simple continuous pattern with 0 polydioxanone suture. The subcutaneous tissue was closed in a simple continuous pattern with 3-0 polydioxanone suture. The skin was closed with surgical staples.

Figure 5. The foreign body, a stick, measured 6 cm.
After surgery, the flank abscess appeared to communicate with the retroperitoneal draining tract because there was a large amount of caseous, mucopurulent discharge and lavage fluids exiting the flank sinus. Postoperative topical cleansing was performed on an as-needed basis with 4-in-x-4-in gauze and a dilute chlorhexidine solution. Sulfamethoxazole-trimethoprim (66 mg/kg and 13 mg/kg orally b.i.d.) and amoxicillin (22 mg/kg orally t.i.d.) were instituted after surgery, pending culture and antimicrobial sensitivity testing. A Schirmer tear test, performed before sulfamethoxazole-trimethoprim therapy was instituted, revealed normal tear function in both eyes.
Bacterial culture and antimicrobial sensitivity testing results indicated a mixed population of a few diphtheroid bacteria, an undetermined amount of gram-negative anaerobic rods, and an undetermined number of gram-positive anaerobic cocci. Bacteria could not be isolated and identified from culture. The culture results for mycoplasma and fungi were negative.
By two days after surgery, the skin tracts had sealed (Figure 6). The dog was then discharged, and antimicrobial therapy was continued as prescribed postoperatively for a total of 28 days. Eleven months after presentation, the owner reported that the dog was doing well. There had been no drainage or swelling in the flank, and the dog's body condition had returned to normal.

Figure 6. The patient two days after recovery from surgery. Inset: A close-up of the dog's left flank. The drainage has stopped, and the cutaneous tracts have started to heal.
Discussion
Differential diagnoses for chronic draining tracts include infection with pyogenic organisms, a foreign body nidus, bone sequestra, neoplasia, fungal infections, and osteomyelitis.1,2 Because chronic microbial infection is associated with or a direct cause of chronic draining tracts, it stands to reason that chronic draining tracts may be complicated or perpetuated by immune-mediated disease and concurrent immune suppression (hyperadrenocorticism, immunosuppressive therapy, or other concurrent systemic disease that has exhausted the immune system). Abscess formation that is nonresponsive or is initially responsive but relapses after treatment is discontinued should alert the clinician to the presence of one or more of the above pathologic processes.
Typical bacteria associated with abscesses include Staphylococcus species, Streptococcus species, facultative and obligate anaerobes, Actinomyces pyogenes (recently reclassified as Arcanobacter pyogenes), Bacteroides species, Escherichia coli, Enterobacter species, Pasteurella multocida, Proteus mirabilis, and Pseudomonas aeruginosa.1,3 Although less frequently diagnosed, mycoplasma infections must also be ruled out. The cause of the abscess and the character of the discharge must be considered when determining the most likely pathogen and most appropriate antimicrobial therapy. In this case, Actinomyces species and Nocardia species were suspected because of the caseous discharge from the flank wound and the propensity for these microbes to produce exudates containing sulfur granules. In addition, Actinomyces species and Nocardia species are often found in mixed populations of infections and are commonly associated with foreign bodies.
A foreign body can be identified by using palpation, radiography (plain and computed tomography), fistulography, ultrasonography, magnetic resonance imaging, or surgical exploration.1,2,4-6 Plain radiography should be performed first to rule out radiopaque foreign bodies, involvement or infection of osseous structures (periosteal reaction), gas-containing radiolucency, and soft tissue swelling.4,7 Ultrasonography has been shown to be an effective tool for diagnosing nonradiopaque foreign bodies in animals.1,4,8,9 In addition, although fistulography and sinography are effective, they have less sensitivity for diagnosing foreign bodies. 2,4,5
A CBC, a serum chemistry profile, cytology, bacterial and fungal culture, and antimicrobial sensitivity testing should be used as baseline tests for an animal presenting with an abscess of unknown origin. Histology should also be performed to rule out a neoplastic process.
Cutaneous sinus tracts from the lumbar, paralumbar, and caudal flank in animals are commonly associated with peritoneal or retroperitoneal foreign bodies.7 This association may occur because the hypaxial musculature converges in this region, the body tends to wall off and exteriorize local inflammatory insults, and draining fluid tends to follow the path of least resistance to the skin surface.
Wood fragments, thorns, cocktail sticks, grass, grass awns, surgical swabs, and nonabsorbable sutures have all been reported as common foreign bodies that lead to chronic draining sinus tracts.2,7 The average duration of clinical signs associated with chronic draining tracts before referral to a surgical specialist is 9.8 months (range = 0.5 to 33 months).2 And the referring veterinarian performs an average of two (range = one to five) surgical procedures before referral to a surgical specialist.2
In the human medical literature, an abundance of information regarding spontaneous cutaneous skin fistulae resulting from a retroperitoneal or abdominal nidus is available. Typically these draining tracts will exit the patient in the caudal flank,10-25 chest wall,26 gluteal area,27 or thigh.28 The nidus for infection in these reported cases in people included a retroperitoneal pseudomyxoma,10 gastrointestinal tract disease,12,13,17,25,27-29 skeletal disease,12,13,15,23 urinary or reproductive tract disease,12,13,16,23,24 surgical complication after laparoscopic cholecystectomy,14,19 surgical complication after hemorrhoidectomy,11 idiopathic disease,18 and unspecified disease.20,22 People with psoas or flank abscesses are often characterized as having a psoas sign—a triad of fever, flank swelling or pain, and pain or decreased range of motion in the ipsilateral hip.
Summary
The clinical approach to chronic draining tracts and skin sinuses should include a thorough history, a physical examination, blood evaluation, cytology, histology, bacterial and fungal culture and antimicrobial sensitivity testing (aerobic and anaerobic), radiography, and advanced imaging if appropriate. A flank abscess in animals should evoke high clinical suspicion of a retroperitoneal or dorsal abdominal musculature nidus as a source of the cutaneous fistula or flank swelling.
Although not illustrated in its entirety in this case, the psoas triad (seen in people) of fever, flank swelling or pain, and pain or decreased range of motion of the ipsilateral coxofemoral joint can further increase clinical suspicion of a flank abscess and a concurrent retroperitoneal or abdominal nidus. This case also illustrates the use of intraoperative ultrasonography to help identify, locate, and remove a deep retroperitoneal foreign body.
This case report was provided by Mathieu M. Glassman, VMD, and Daniel D. Smeak, DVM, DACVS, Department of Veterinary Clinical Sciences, College of Veterinary Medicine, The Ohio State University, Columbus, Ohio. Dr. Glassmanâs present address is Friendship Hospital for Animals, 4105 Brandywine St. N.W., Washington, D.C. 20016.
REFERENCES
1. Staudte KL, Hopper BJ, Gibson NR, et al. Use of ultrasonography to facilitate surgical removal of non-enteric foreign bodies in 17 dogs. J Small Anim Pract 2004;45:395-400.
2. Lamb CR, White RN, McEvoy FJ. Sinography in the investigation of draining tracts in small animals: retrospective review of 25 cases. Vet Surg 1994;23:129-134.
3. Aucoin D. Target: the antimicrobial reference guide to effective treatment. Port Huron, Mich: North American Compendiums Inc, 2004.
4. Armbrust LJ, Biller DS, Radlinsky MG, et al. Ultrasonographic diagnosis of foreign bodies associated with chronic draining tracts and abscesses in dogs. Vet Radiol Ultrasound 2003;44:66-70.
5. Duffey MH, Léveillé R, Smeak DD. What is your diagnosis? Nonradiopaque linear foreign body as a probable cause of a skin sinus. J Am Vet Med Assoc 1998;213:1557-1558.
6. Adams R, Nixon A, Hager D. Use of intraoperative ultrasonography to identify a cervical foreign body. A case report. Vet Surg 1987;16:384-388.
7. Kirby BM. Peritoneum and peritoneal cavity. In: Slatter D, ed. Textbook of small animal surgery. 3rd ed. Philadelphia, Pa: WB Saunders Co, 2003;440-441.
8. Cartee RE, Rumph PF. Ultrasonographic detection of fistulous tracts and foreign objects in muscles of horses. J Am Vet Med Assoc 1984;184:1127-1132.
9. Shah ZR, Crass JR, Oravec DC, et al. Ultrasonographic detection of foreign bodies in soft tissues using turkey muscle as a model. Vet Radiol Ultrasound 1992;33:94-100.
10. Koizumi J, Noguchi H. Pseudomyxoma retroperitonei with spontaneous skin fistula. Abdom Imaging 1999;24:193-195.
11. Pryor JP, Piotrowski E, Seltzer CW, et al. Early diagnosis of retroperitoneal necrotizing fasciitis. Crit Care Med 2001;29:1071-1073.
12. Zissin R, Gayer G, Kots E, et al. Illiopsoas abscess: a report of 24 patients diagnosed by CT. Abdom Imaging 2001;26:533-539.
13. Hamano S, Kiyoshima K, Nakatsu H, et al. Pyogenic psoas abscess: difficulty in early diagnosis. Urol Int 2003;71:178-183.
14. Papasavas PK, Caushaj PF, Gagné DJ. Spilled gallstones after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A 2002;12:383-386.
15. Fitoz S, Atasoy C, Yagmurlu A, et al. Psoas abscess secondary to tuberculous lymphadenopathy: case report. Abdom Imaging 2001;26:323-324.
16. Milam MR, Schultenover SJ, Crispens M, et al. Retroperitoneal fibrosis secondary to actinomycosis with no intrauterine device. Obstet Gynecol 2004;104:1134-1136.
17. Kobayashi H, Sakurai Y, Shoji M, et al. Psoas abscess and cellulitis of the right gluteal region resulting from carcinoma of the cecum. J Gastroenterol 2001;36:623-628.
18. Iwaki H, Mori H, Kajita Y, et al. Giant psoas abscess with aggressive extension: report of a case. [Japanese] Hinyokika Kiyo 1999;45:835-837.
19. Brueggemeyer MT, Saba AK, Thibodeaux LC. Abscess formation following spilled gallstones during laparoscopic cholecystectomy. JSLS 1997;1:145-152.
20. Chern CH, Hu SC, Kao WF, et al. Psoas abscess: making an early diagnosis in the ED. Am J Emerg Med 1997;15:83-88.
21. Hoffer FA, Shamberger RC, Teele RL. Ilio-psoas abscess: diagnosis and management. Pediatr Radiol 1987;17:23-27.
22. Lee YT, Lee CM, Su SC, et al. Psoas abscess: a 10 year review. J Microbiol Immunol Infect 1999;32:40-46.
23. Penado S, Espina B, Francisco Campo J. Abscess of the psoas muscle. Description of a series of 23 cases. [Spanish] Enferm Infecc Microbiol Clin 2001;19:257-160.
24. Sahin H, Bircan MK, Gedik A. A retroperitoneal abscess with cutaneous fistula developed after stercoral fistula operation: a case report. Int Urol Nephrol 1998;30:39-40.
25. Distefano M, Bonanno G, Russo A. Biliocutaneous fistula following biliary stent migration. Endoscopy 2001;33:97.
26. Poulos J, Johnson SR, Conrad P, et al. Dome-shaped lesion on chest radiograph: retroperitoneal abscess dissecting through the posterior chest wall. South Med J 1994;87:77-80.
27. Sinha DD, Sharma C, Gupta V, et al. Post-traumatic retroperitoneal colonic injury presenting as gluteal abscess. Indian J Gastroenterol 2004;23:151-152.
28. Lee SS, Chan YS, Chen CY, et al. Non-tuberculous cold abscess of the psoas muscle—an unusual manifestation of colocutaneous fistula. Arch Orthop Trauma Surg 2000;120:224-225.
29. Kim CJ, Kato K, Yoshiki T, et al. Intractable duodenocutaneous fistula after nephrectomy for stone pyonephrosis: report of a case. [Japanese] Hinyokika Kiyo 2003;49:547-550.