Clinical Exposures: Preovulatory stasis and dystocia in oviparous lizards
A 2-year-old intact female bearded dragon (Pogona vitticeps) was presented for evaluation of weakness and anorexia.
A 2-year-old intact female bearded dragon (Pogona vitticeps) was presented to Tufts University's Cummings School of Veterinary Medicine for evaluation of weakness and anorexia of one day's duration. On initial presentation, the bearded dragon was lethargic, with a distended coelom and pale mucous membranes. The results of the initial blood work revealed severe anemia; the patient's packed cell volume was 6% (reference range = 24% to 36%).1 A dorsoventral radiograph revealed multiple rounded (about 1 cm in diameter), coalescing, soft tissue opacities throughout the caudal coelomic cavity (Figure 1). Preovulatory stasis was tentatively diagnosed. Coelomic ultrasonography was recommended to evaluate the abdominal opacities, but the owner declined further investigation.
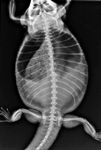
1. A dorsoventral radiograph of a bearded dragon with preovulatory stasis. Note the multiple rounded coalescing soft tissue opacities throughout the caudal half of the coelomic cavity, consistent with follicles.
The bearded dragon was hospitalized, an intraosseous catheter was placed in the left tibia, and fluid therapy was initiated. Reptile Ringer's solution (i.e. one part lactated Ringer's solution, two parts 2.5% dextrose/0.45% saline solution)1 was administered at a rate of 25 ml/kg/day. The lizard was placed in a heated, humidified cage with an ambient temperature of 85 F (29.5 C). The reptile became increasingly lethargic and weak over the next 12 hours. Because there was no improvement and the lizard's condition carried a poor prognosis, the owner elected euthanasia and a postmortem examination.
Necropsy revealed that the coelomic cavity contained about 10 ml of serosanguineous fluid. Each ovary contained about 30 enlarged (1-cm-diameter), round, bright-yellow vitellogenic follicles (Figure 2). The liver was diffusely pale-yellow, a typical physiologic finding in species that lay large numbers of eggs.
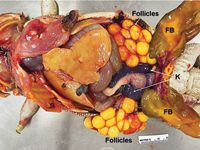
2. Postmortem examination of the coelomic cavity of a female bearded dragon with preovulatory stasis. The liver shows normal fatty change associated with vitellogenesis. Both ovaries contain numerous round, bright-yellow, nonshelled vitellogenic follicles. Part of the small and large intestines, both kidneys (K), and the fat bodies (FB) are also shown.
A histologic examination of the ovaries revealed that the follicles contained large amounts of bright eosinophilic proteinaceous yolk surrounded by several layers of flattened epithelial cells. Bone marrow, collected from the proximal aspects of the humerus and femur, was atrophic and composed mostly of sheets of intact adipocytes with rare hematopoietic cells, explaining the low hematocrit. The liver showed diffuse fatty change. The clinical history and gross and histologic findings were most consistent with a diagnosis of preovulatory stasis (follicular stasis). Marrow aplasia, which was seen in this lizard, is not a typical finding with this condition but may have been caused by estrogen toxicosis, as a result of the high estrogen concentrations associated with follicular growth. While estrogen-induced marrow aplasia has been well-documented in dogs and ferrets,2 this effect has not been reported in reptiles.
DISCUSSION
Reproductive disorders are common in captive reptiles (lizards, chelonians [tortoises, turtles], and snakes).3 The most common reproductive disorders in female reptiles are preovulatory follicular stasis and postovulatory egg stasis (dystocia), which are often related to inappropriate husbandry (malnutrition, an inadequate nesting site, inappropriate temperature and humidity, or the absence of a UVA and UVB source).1 Sexual maturity in reptiles is determined predominantly by body size, though age plays a secondary role. In captivity, reptiles can reach sexual maturity much earlier than in natural environments,1 so preovulatory stasis may be seen in relatively young (< 1 year old) captive reptiles. The presence of a male in the same terrarium does not affect the incidence of preovulatory stasis. Preovulatory stasis is not seen in wild lizards.
Normal follicular development in reptiles
An important phase in follicle development in reptiles is vitellogenesis (i.e. formation of yolk in the liver, with subsequent deposition around the ova within each follicle).4 Estrogens stimulate the liver to convert the adipose tissue and dietary lipids into vitellogenin,4 which is selectively absorbed from the circulation by the follicles. During this stage, follicles can reach up to 100 times their original size. The liver also dramatically enlarges and becomes bright yellow.
Large amounts of calcium, essential for shell formation, are also added to the follicles. During this phase, total serum calcium concentrations are as much as two to four times higher than normal.5 Most of the serum calcium is bound to serum proteins, including vitellogenins and egg yolk lipoproteins. Ionized calcium may also increase slightly.
The follicle becomes an egg after the deposition of albumin and formation of a shell inside the oviduct. After ovulation, there is little exchange of nutrients between the female and the egg.
Causes of preovulatory stasis and dystocia in reptiles
An interruption in follicular development can result in preovulatory stasis. The cause of preovulatory stasis is often difficult to determine, though a husbandry-related problem is most common. For example, if the animal lacks exposure to UVB rays and has inadequate calcium supplementation,3 it may not be able to complete ova development and subsequently cannot ovulate. Large amounts of calcium in the yolk serve the neonatal lizard's needs,4 and calcium is necessary for shell development. In this case, the cause of the condition could not be determined.
Dystocia occurs when the animal ovulates and develops eggs (Figure 3) but is unable to pass them from the oviduct. This condition may arise because of a problem with the egg itself (malpositioning, malformation, or rupture) or a problem with the female such as a pelvic or oviductal malformation or masses not related to the reproductive tract such as abscesses or urinary bladder calculi.3 A female that has had a previous dystocia is predisposed to having it occur again as a result of scar tissue from previous salpingotomies.6 Other causes include poor physical condition (captive reptiles may not have the muscle tone needed to lay their eggs), dehydration, low environmental temperatures, low humidity, and the lack of a suitable area for nest digging.3 Although oviductal infection is a potential cause, infectious etiologies are not well-documented or well-understood in reptiles.
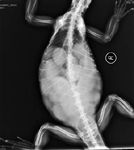
3. A dorsoventral radiograph of a green iguana with numerous shelled eggs in the abdomen. Note that the shelled eggs are more radiopaque, with more distinct outlines, than the nonshelled follicles shown in Figure 1.
Diagnosis
Diagnosing preovulatory stasis or dystocia can be problematic because it can be difficult to differentiate between true reproductive system pathology and the normal phases of egg development. The presence of eggs in the coelom can be easily detected by ultrasonography, which allows the distinction between shelled eggs and preovulatory follicles. (Shelled eggs are oblong rather than rounded and are arranged in a line rather than in clusters. Shelled eggs have a hypoechoic center and a hyperechoic perimeter [shell].7 ) Radiography can be useful in determining egg positioning, but it can be difficult to detect nonshelled eggs, and if visible, it may not be possible to determine if the eggs have well-developed shells. Lizard eggs may be detected by abdominal palpation by experienced practitioners, but it is best to confirm their presence by abdominal radiography or ultrasonography because other masses could be mistaken for eggs.
After confirming the presence of eggs, the next step is to determine whether the animal is undergoing the normal physiologic order of events that will lead to egg deposition or if it is experiencing preovulatory stasis or dystocia. Obtaining a good history, observing clinical signs, and performing supplemental diagnostic tests are fundamental to an accurate diagnosis. An extremely swollen abdomen is common in normal, gravid female lizards, and some females will not eat for several weeks before laying eggs. However, a normal, gravid female is active and alert. Acute onset of lethargy or unresponsiveness, tremors, weight loss, or a dull skin color are important abnormalities.8 Carefully question the owner about all husbandry practices to detect possible husbandry-related problems.
If the reptile has already passed several eggs and additional eggs remain in the coelomic cavity, dystocia is most likely, especially if the animal is depressed and reluctant to move. If the animal has not passed any eggs and is depressed and abdominal palpation and radiography or ultrasonography confirm that nonshelled eggs are in the coelom, preovulatory stasis is most likely. In some cases, it can be difficult to discern between a nonshelled and a shelled egg, so dystocia should remain on the list of differential diagnoses.
Treatment
Ovariectomy is the treatment for preovulatory follicular stasis (Figure 4).6 In rare species, follicle removal can be attempted but only by surgeons with experience performing this procedure. If follicles fail to ovulate and are not reabsorbed or surgically removed, they may coalesce, forming a large friable yolk mass. If this mass ruptures, it can result in severe inflammation and possible death. In this case, the owner declined surgery because of the lizard's poor prognosis.
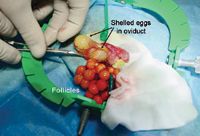
4. Surgical removal of the ovaries with multiple follicles. Shelled eggs are present within the oviduct. The shelled eggs were also removed but not the oviduct. (Image courtesy of Paolo Selleri, Dr. med. Vet., Centro Veterinario Specialistico, Rome Italy.)
With dystocia, if the animal is not severely debilitated, medical therapy and physical massage to assist in egg passage can be attempted.3 However, physical massage is not recommended in lizards, especially for inexperienced veterinarians. The eggs may rupture and cause serious pathology (such as egg yolk coelomitis). Administering calcium gluconate, subcutaneous fluids, and oxytocin and providing a warm and humid environment may help the reptile pass the shelled eggs. However, it is important to first rule out preovulatory stasis since medical therapy with calcium and oxytocin will be ineffective if the lizard has this condition. Surgery (to remove the shelled eggs and the ovaries) may be necessary if medical therapy fails. If surgery is required, it is advantageous to first stabilize the animal. A complete blood count and serum chemistry profile are important in ruling out infection or organ failure.
CONCLUSION
The reproductive diseases discussed above are common in captive female reptiles, but obtaining an accurate diagnosis and resolving the problem can be difficult for practitioners with little experience in reptile medicine. Prevention remains the best approach, so properly educating reptile owners is essential. Elective ovariectomy of lizards and chelonians should be considered but is best performed by exotic-animal specialists.
This case report was provided by Raffaele Melidone, Dr. med. vet.; Joyce S. Knoll, VMD, PhD, DACVP; and Nicola Parry, BSc, MSc, BVSc, DACVP, Department of Biomedical Sciences, Cummings School of Veterinary Medicine, Tufts University, North Grafton, MA 01536.
REFERENCES
1. Campbell TW. Clinical chemistry of reptiles. In: Thrall MA, Baker DC, Campbell TW, et al. eds. Veterinary hematology and clinical chemistry. Philadephia, Pa: Lippincott Williams & Wilkins, 2004;493-498.
2. Hart JE. Endocrine pathology of estrogens: species differences. Pharmacol Ther 1990;47(2):203-218.
3. DeNardo D. Dystocias. In: Reptile medicine and surgery. 2nd ed. St. Louis, Mo: Elsevier Saunders, 2006;787-792.
4. DeNardo D. Reproductive biology. In: Reptile medicine and surgery. 2nd ed. St. Louis, Mo: Elsevier Saunders, 2006;376-390.
5. Diethelm G. Reptiles. In: Exotic animal formulary. 3rd ed. St. Louis, Mo: Elsevier Saunders, 2005;53-131.
6. Mader DR, Bennett A, et al. Surgery. In: Reptile medicine and surgery. 2nd ed. St. Louis, Mo: Elsevier Saunders, 2006;594.
7. Stetter MD. Ultrasonography. In: Reptile medicine and surgery. 2nd ed. St. Louis, Mo: Elsevier Saunders, 2006;665-674.
8. Wissman MA. When to intervene in reptile parturition. Veterinary Practice News http://www.veterinarypracticenews.com/vet-dept/avian-exotic-dept/reptile-breeding.aspx. Accessed October 20, 2008.