Clinical Exposures: Vertebral articular process hypertrophy causing spinal cord compression in a Great Dane
A 6-year-old castrated male Great Dane was presented to the Cornell University Hospital for Animals for evaluation of pelvic limb ataxia and intermittent fecal incontinence.
A 6-year-old castrated male Great Dane was presented to the Cornell University Hospital for Animals for evaluation of pelvic limb ataxia and intermittent fecal incontinence. The owner reported that the ataxia had first been noticed when the dog was about 1.5 years old and had slowly progressed. Fecal incontinence had been present for about two months. The owner noted that, in addition to defecating normally, about every two days the dog had accidents in the house during which it appeared unaware that it was defecating.
The patient had been evaluated at another hospital about seven months previously for the pelvic limb ataxia. At that time, caudal cervical spondylomyelopathy had been suspected and magnetic resonance imaging had been performed, which revealed no evidence of caudal cervical spondylomyelopathy. Cerebrospinal fluid analysis had also been performed at that time, which revealed no abnormalities.
EXAMINATIONS AND DIAGNOSTIC TESTS
On presentation, the dog was quiet, alert, and responsive. The only abnormality identified on physical examination was hyperemia and nail wear of the fifth digit of both pelvic limbs. No abnormalities were identified on rectal examination.
The dog exhibited moderate pelvic limb general proprioceptive ataxia when walking. A neurologic examination revealed bilateral crossed extensor reflexes and upper motor neuron paresis in the pelvic limbs and decreased proprioceptive positioning reactions in both pelvic limbs. The patient ambulated well with the pelvic limbs but was moderately ataxic and occasionally scuffed the dorsum of the toes on either limb. Spinal reflexes (with the exception of the crossed extensor reflex) and muscle tone were normal in both pelvic limbs. The thoracic limbs were normal, as was the perineal reflex.
We localized the patient's lesion to the T3-L3 spinal cord segments and decided to evaluate the thoracolumbar spine with magnetic resonance imaging. The dog's CBC and serum chemistry profile results were within reference ranges, so the dog was anesthetized. At the right synovial articulation of L3-L4, the articular processes (facets) were enlarged and bulbous, having substantial new bone formation with minimal associated surrounding soft tissue. This new bone formation expanded into the vertebral canal, causing mild right dorsolateral compression of the spinal cord (Figures 1A & 1B).
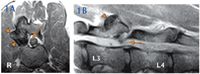
Figure: 1A & 1B. Axial T2-weighted and sagittal T1-weighted magnetic resonance images of the midlumbar region of the dog in this report. At L3-L4, the right articular processes are hypertrophied (arrowheads) causing mild, right, dorsolateral spinal cord compression (thin arrows).
Immediately after the magnetic resonance imaging, we evaluated the abnormal articular processes by using computed tomography. Three-dimensional reconstruction revealed a large, smooth bridging bone formation at the right synovial articulation of L3-L4, along with mild deformation of the right pedicle and lamina of L4 (Figures 2A & 2B).

Figure: 2A & 2B. Computed tomography three-dimensional reconstructions of the midlumbar vertebrae of the dog in this report viewed from the right craniodorsally (2A) and dorsally (2B). At L3-L4, the hypertrophy of the right articular processes appears as bulbous, smooth, new bone formation bridging the synovial joint. Additionally, the spinous process of L4 is mildly deviated to the left.
TREATMENT AND OUTCOME
The following day, a modified hemi laminectomy was performed at L3-L4, and all compressive material was removed. Small fragments of the lesion were submitted for aerobic and anaerobic bacterial culture. The remainder of the collected fragments were submitted for histologic examination. Intravenous cefazolin (22 mg/kg) was administered every 90 minutes during surgery. For the initial 24 hours after surgery, intravenous fentanyl (3 μg/kg/hr) was administered by constant-rate infusion for analgesia.
The patient recovered well, and at the time of discharge 72 hours after surgery, the dog was ambulatory in the pelvic limbs with mild general proprioceptive ataxia and upper motor neuron paresis. We prescribed an approximately two-week tapering schedule of oral prednisone (0.5 mg/kg once a day for five days, then 0.25 mg/kg once a day for five days, then 0.25 mg/kg once a day every 48 hours for five days). The patient also received oral cephalexin (22 mg/kg orally t.i.d.) for 14 days postoperatively. We recommended slow leash walks for two weeks, with a gradual return to normal activity over the ensuing two to three months.
The aerobic and anaerobic bacterial cultures yielded no growth. Histologic findings included highly reactive periosteal proliferation, irregular and degenerative articular cartilage, reactive synovial cells, and regenerating chondrocytes. The morphologic diagnosis was severe locally extensive periosteal proliferation with moderate cartilaginous hyperplasia, consistent with degenerative joint disease.
On reexamination four weeks after surgery, the dog exhibited minimal pelvic limb ataxia. The owner also reported that the dog had displayed only two episodes of fecal incontinence since surgery.
DISCUSSION
Myelopathy due to vertebral articular process, or facet, hypertrophy and subsequent spinal cord compression is infrequently described in the veterinary literature.1,2 It is generally thought that malarticulation between the cranial and caudal vertebral articular processes leads to joint instability and progressive hypertrophy. Two possible reasons for such malarticulation are prior trauma or a congenital malformation, the latter of which is more likely.1-3
Malarticulation theories
Some researchers have theorized that caudal vertebral articular process dysplasia, based on imaging findings in four dogs, offers a reasonable explanation for vertebral articular process hypertrophy.2 This theory proposes that congenital hypoplasia or aplasia of the caudal vertebral articular process leads to chronic instability of the affected intervertebral space. Over time, progressive hypertrophy of the cranial vertebral articular process, as well as adjacent soft tissue structures (e.g. synovium, ligamentum flavum), leads to vertebral canal impingement and spinal cord compression.2 This explanation likely applies to the dog in this case and is consistent with the histologic findings. Considering the chronicity of the dog's problem and the massive hypertrophy of the vertebral articular process, it is not surprising that a primary defect (i.e. dysplastic caudal vertebral articular process) was not identified on either imaging or histologic examination.
Other possible explanations for articular process hypertrophy include joint infection and abnormal mechanical loading of the joint; the latter phenomenon is suspected to be due to altered intervertebral joint movement secondary to degenerative intervertebral disk disease.3 Breed-associated vertebral articular process hypertrophy has been described in Shiloh shepherds and Scottish deerhounds.1,3 In a family of Shiloh shepherds, hypertrophied vertebral articular processes in the thoracolumbar region, causing spinal cord compression, were described.1 In Scottish deerhounds, arthrosis of the synovial articulations at C2-C3 was associated with cervical hyperesthesia, in the absence of myelographic evidence of spinal cord compression.3 To the our knowledge, there are no other reports of vertebral articular process hypertrophy-associated myelopathy in Great Danes.
Unusual features of this case
With regard to the location of the spinal cord lesion, our patient's clinical presentation had two unusual features. First, in most dogs, the spinal cord lower motor neuron cell bodies supplying the femoral and part of the sciatic nerves are located at the L3-L4 vertebral level.4 Despite the location of the hypertrophied articular processes at L3-L4, the dog in this report displayed clinical signs consistent with general proprioceptive ataxia due to upper motor neuron dysfunction. One possibility for this apparent discrepancy is individual anatomical variation. Another possibility relates to the nature of the lesion itself. The hypertrophied articular processes caused mild compression of the dorsal and lateral portions of the spinal cord, regions occupied primarily by ascending sensory tracts. The lower motor neurons in this region likely experienced minimal compression from the abnormal synovial articulation, with most of the compression directed toward sensory proprioceptive white matter tracts.
The other unusual aspect of our patient's clinical presentation was fecal incontinence in the absence of a lesion in the region of the sacral spinal cord segments. Fecal incontinence is commonly associated with lesions in the sacral spinal cord segments or associated spinal nerve roots, as these structures innervate the anal sphincter musculature.4 Fecal incontinence is a recognized complication of degenerative lumbosacral stenosis in dogs and is associated with a poor to guarded prognosis for postoperative resolution if present for more than one month.5,6
Fecal incontinence associated with upper motor neuron spinal cord lesions has recently been described in seven dogs.7 Similar to the dog in our case report, all seven dogs had lesions compressing the dorsal aspect of the spinal cord, and all had normal perineal sensation and perineal (anal sphincter) reflexes. It is theorized that fecal incontinence associated with upper motor neuron spinal cord lesions is due to interruption of ascending sensory information from the rectum, interruption of descending inhibitory influence on the sacral defecation reflex, or a combination of the two.7 In the report of seven dogs with fecal incontinence, the incontinence resolved in four of the five dogs that underwent decompressive surgery, despite a history of incontinence ranging from three months to three years.7 Although case numbers are still low, it appears that fecal incontinence associated with upper motor neuron dysfunction may more likely resolve after surgical decompression than will fecal incontinence associated with lower motor neuron dysfunction.
CONCLUSION
Clinicians should be aware that vertebral articular process hypertrophy can cause myelopathy in dogs. In addition, fecal incontinence can occur secondary to upper motor neuron lesions (i.e. cranial to the sacral spinal cord segments), and this incontinence may be ame na ble to surgical intervention.
This case report was provided by Curtis W. Dewey, DVM, MS, DACVIM (neurology), DACVS; Ursula Krotscheck, DVM, DACVS; Kevin Winegardner, DVM; and Maureen Vaillancourt, DVM, Department of Clinical Sciences, College of Veterinary Medicine, Cornell University, Ithaca, NY 14853.
REFERENCES
1. McDonnell JJ, Knowles KE, deLahunta A, et al. Thoracolumbar spinal cord compression due to vertebral process degenerative joint disease in a family of Shiloh Shepherd dogs. J Vet Intern Med 2003;17:530-537.
2. Penderis J, Schwarz T, McConnell JF, et al. Dysplasia of the caudal vertebral articular facets in four dogs: results of radio graphic, myelographic and magnetic resonance imaging investigations. Vet Rec 2005;156:601-605.
3. Kinzel S, Hein S, Buecker A, et al. Diagnosis and treatment of arthrosis of cervical articular facet joints in Scottish Deerhounds: 9 cases (1988-2002). J Am Vet Med Assoc 2003;223:1311-1315.
4. Dewey CW. Functional and dysfunctional neuroanatomy: the key to lesion localization. In: Dewey CW, ed. A practical guide to canine and feline neurology. Ames: Iowa State Press (Blackwell Publishing), 2003;3-30.
5. Dewey CW. Disorders of the cauda equina. In: Dewey CW, ed. A practical guide to canine and feline neurology. Ames: Iowa State Press (Blackwell Publishing), 2003;337-355.
6. Danielsson F, Sjostrom L. Surgical treatment of degenerative lumbosacral stenosis in dogs. Vet Surg 1999;28:91-98.
7. Chen AV, Bagley RS, West CL, et al. Fecal incontinence and spinal cord abnormalities in seven dogs. J Am Vet Med Assoc 2005;227:1945-1951.