Clinical Rounds: Battling a Labrador's oral malignant melanoma
This team of experts stepped in to help extend the dog's life with targeted treatment. What can you learn from their approach to help your patients?
OVERVIEW
Oral malignant melanoma is the most common tumor of the oral cavity in dogs.1-4 Predisposed breeds include Scottish terriers, golden retrievers, poodles, and dachshunds.2-4 The metastatic rate is high, with more than 80% of patients developing metastasis.5 Common metastatic sites include draining lymph nodes and the lungs.5 About 40% of lymph nodes that are normal on palpation have evidence of metastasis on fine-needle aspiration.6 The median survival time is eight to 10 months, but it can vary depending on the tumor's location and size and the presence of bone lysis.
CASE PRESENTATION
A 12-year-old castrated male black Labrador retriever was presented to the University of Tennessee Veterinary Medical Center for staging and treatment of a right maxillary oral malignant melanoma. The owners noted that the patient was having difficulty eating and that they saw drops of blood around the food bowl about one month before presentation. Three weeks before presentation, the referring veterinarian performed a debulking surgery, removing as much of the gross disease as was possible without a maxillectomy, and submitted a biopsy sample, which confirmed a diagnosis of oral malignant melanoma.
Physical examination and tumor staging
On physical examination, multiple, soft subcutaneous masses were noted on the dog's trunk, which were aspirated as lipomas. An awake oral examination revealed a pink, raised, multi-lobulated mass on the right hard palate, extending from the first premolar caudally to the third premolar. The mass extended medially to about 0.5 cm beyond midline. All of the peripheral lymph nodes palpated normally.
Staging tests consisted of a complete blood cell count, a serum chemistry profile, a urinalysis, three-view thoracic radiography, two-view abdominal radiography, abdominal ultrasonography, and cytologic examination of fine-needle aspirates of the right and left mandibular and prescapular lymph nodes.
All of the test results were normal, with no obvious evidence of metastasis. A computed tomography (CT) scan from the tip of the nares caudally to the area of the second cervical vertebral body was performed to evaluate the extent of the tumor and assess the lymph nodes in the head and cranial neck.
The CT scan confirmed that the tumor extended from the first premolar caudally to the third premolar and that it crossed midline on the hard palate. The CT scan also revealed lysis of the right hard palate and portions of the right maxilla. The tumor extended through the hard palate and into the right nasal cavity, causing almost complete occlusion of the right nasal passage. The lymph nodes appeared normal in size and contrast enhancement on the CT scan.
Treatment
A surgery consult confirmed that the tumor was too large to be completely excised, and hypofractionated radiation therapy was recommended. Treatment consisted of four weekly 8 Gy fractions to the primary tumor site and draining lymph nodes. We also recommended a canine melanoma vaccine (Oncept—Merial) once every other week for four treatments to control potential metastatic disease.
The patient finished radiation therapy and the four initial treatments with the melanoma vaccine without any serious clinical effects. The local tumor responded well to radiation therapy, with a reduction in size of about 50%.
Follow-up
One month after finishing the melanoma vaccine (seven weeks after radiation therapy) the patient presented for follow-up three-view thoracic radiography. The owners reported that the patient was doing well at home but noted halitosis.
A physical examination revealed an enlarged right mandibular lymph node and infected necrotic tissue associated with the right hard palate, which was the original tumor location. Thoracic radiographs showed a soft tissue nodule in the right middle lung lobe, and aspirates of the right mandibular lymph node revealed neoplastic cells consistent with malignant melanoma. The patient was sedated, and the necrotic tissue was débrided and cleaned. Amoxicillin trihydrate-clavulanate potassium was prescribed, and we recommended the patient return the next week for chemotherapy if the infection was under control.
The following week, the infection appeared to be well-controlled, so we initiated carboplatin chemotherapy at a dose of 250 mg/m2. While the recommended dose of carboplatin is 275 to 300 mg/m2, the oncologists at the University of Tennessee have seen a high number of cases develop marked neutropenias and sepsis when carboplatin is initiated at this dose. Therefore, we treat patients at a dose of 250 to 275 mg/m2. The patient tolerated the carboplatin therapy well and received a second treatment three weeks later.
When the dog returned for restaging three weeks after the second carboplatin therapy, thoracic radiographs revealed multiple soft tissue nodules throughout the lung lobes. Since the patient continued to do well at home, the owner opted to stop therapy at that point and monitor the patient's quality of life.
The patient was humanely euthanized because of poor quality of life three months later, nine months after diagnosis.
CLINICAL PATHOLOGY PERSPECTIVE
Jennifer Scruggs, DVM
Definitive diagnosis of malignant melanoma based on fine-needle aspiration is often difficult. Although samples are typically of good cellularity, cytomorphology of both benign and malignant lesions can exhibit marked variation and may range from round to epithelioid or spindle-shaped.

Jennifer Scruggs, DVM
The presence of cytoplasmic pigment (i.e. fine to coarse, brown, black, or green granules) may help identify the cells as melanocytic in origin; however, it is not always possible to distinguish melanin from other pigment (especially hemosiderin) without the benefit of special stains. Pigmentation of melanomas can vary both within and among tumors. Some lesions are amelanotic; therefore, the lack of melanin granules does not exclude melanoma from a differential list. Close inspection of nuclei may provide some insight into the malignant potential of the lesion—increased anisokaryosis, prominent and pleomorphic nucleoli, and coarse chromatin are most consistent with malignancy (Figure 1).
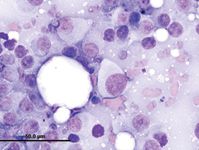
1. A fine-needle aspirate from a lymph node in a dog. The sample contains individual and aggregated round to spindle-shaped cells. The cells have varying amounts of pale-grey, nonpigmented cytoplasm and large, round nuclei. Note the nuclear criteria of malignancy, including moderate to marked anisokaryosis, coarse chromatin, and prominent, multiple, and pleomorphic nucleoli. Rare small lymphocytes and a plasma cell are present.
Diagnosis of metastatic disease in a regional lymph node is not difficult if high numbers of neoplastic cells are present. However, it becomes more challenging when low numbers of neoplastic cells are present given that rare melanocytes may be normally found in healthy lymph nodes. Additionally, melanocytes may be difficult to distinguish from pigment-laden macrophages.
Special stains that may be useful diagnostically for cytologic examination of melanocytic tumors include Fontana-Masson, which may help highlight melanin granules in poorly melanotic lesions, and Prussian blue, which highlights hemosiderin.9
ANATOMIC PATHOLOGY PERSPECTIVE
Julia Lankton, DVM, DACVP
Canine oral malignant melanoma most commonly appears on the gingiva or lips and less often on the cheek, tongue, or palate.10 The degree of melanin pigmentation varies, and tumors can range from heavily pigmented to amelanotic.

Julia Lankton, DVM, DACVP
In addition to cytoplasmic pigmentation, characteristic histologic features include junctional activity (clusters of neoplastic cells at the mucosal-submucosal junction) and nests of neoplastic cells within the mid- to upper epithelium.
Poorly differentiated melanomas can present a diagnostic challenge to pathologists, and immunohistochemistry may be recommended to confirm the cell of origin. Melan-A is the most specific immunohistochemical marker and is also highly sensitive. Neoplastic cells are also typically positive for vimentin, S100, and neuron-specific enolase. Although most canine oral melanomas exhibit aggressive biological behavior, a subset of histologically well differentiated tumors have a prolonged clinical course (Figure 2).11
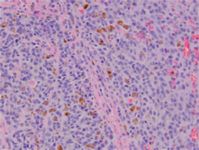
2. The histologic appearance of the tumor in this case. The neoplasm is composed of sheets of pleomorphic cells with a vesicular nucleus, a prominent nucleolus, and a variable amount of cytoplasmic melanin.
SURGICAL PERSPECTIVE
Rachel Seibert, DVM
The treatment of choice in dogs with oral malignant melanoma without distant metastasis is wide surgical excision with margins of at least 2 cm. Recurrence rates range from 15% to 22% in dogs that have undergone complete excision, while those that have undergone incomplete resection have a recurrence rate of up to 65%.12,13 Complete resection of most oral melanomas, with the exception of those confined to the lip margins or tongue, requires partial mandibulectomy or maxillectomy.

Rachel Seibert, DVM
Hemorrhage is the main intraoperative complication, which typically occurs after the transection of the infraorbital, sphenopalatine, or major palatine artery during maxillectomy or the inferior alveolar artery during mandibulectomy. Excessive and potentially life-threatening bleeding may also occur because of disruption of nasal turbinates. Hemorrhage is typically controlled by pressure, vessel ligation, or, in severe cases, temporary carotid artery ligation.14
Mandibulectomies and maxillectomies are closed by suturing mucosa to mucosa in a simple interrupted or continuous pattern with absorbable monofilament suture material. If necessary, flaps may be developed to reduce tension.
Tumors ventral to the orbit may require transection of a portion of the zygomatic arch and the ventral aspect of the orbit, neither of which adds substantially to the overall morbidity.
Labial mucosa may be sutured to preplaced holes drilled in the hard palate to help prevent dehiscence, which is the most common postoperative complication.15 Dehiscence is most common after caudal or central maxillectomies requiring palatal flaps (with a reported incidence of 7% to 33%)4,13,16 and less common after bilateral rostral mandibulectomies when mucosal closure is directly over transected bone ends. Dehiscence can largely be avoided by preventing excessive tension during closure, although it is also associated with incomplete excision, tumor recurrence, exposed bone, excessive use of electrocoagulation, and infection. Often, dehiscence is minor and can be monitored and allowed to heal by second intention.
Other postoperative complications include hypersalivation, difficulty prehending, infection, mandibular drift, malocclusion, inadvertent trauma to tooth roots, subcutaneous emphysema, facial swelling, and oronasal fistula.17 The tongue may hang out from the mouth after hemimandibulectomy. A commissurorrhaphy (surgical closure of the lips further rostrally) may help prevent this.

3. A dog two months after bilateral hemimandibulectomy for an oral tumor.
Surprisingly, most dogs will eat after even radical maxillectomies, and feeding tubes are rarely required. Although the patient's appearance can be drastically altered, the postoperative cosmetic appearance is acceptable to most clients, with 85% of owners being satisfied with their decision to treat their dogs in one study (Figure 3).18
RADIOLOGY PERSPECTIVE
Laura Hammond, DVM
As with most oncology patients, the goals of diagnostic imaging in patients with oral malignant melanoma are to identify the extent of local disease, guide tissue sampling, and screen for metastases and concurrent diseases. Evaluation of the primary oral mass may be performed with either skull radiographs or cross-sectional imaging (CT or magnetic resonance imaging). While radiographs may identify some osseous lesions of the skull, CT is a more sensitive modality for detecting both osseous and soft tissue abnormalities of the head and neck.

Laura Hammond, DVM
CT features of malignant melanoma include variable lysis of adjacent bone, soft tissue swelling, and variable contrast enhancement.7 Contrast-enhanced CT can be used to guide tissue sampling and plan surgical margins of the primary mass since it allows differentiation of typically strongly enhancing tumor tissue from non-enhancing areas (e.g. central necrosis or hemorrhage) or less enhancing adjacent normal tissue.
CT features of lymph node metastasis include heterogeneous contrast enhancement and variable lymphadenomegaly. Mild lymphadenomegaly can be difficult to detect on physical examination but may be readily apparent on CT.
Screening for pulmonary metastases may be accomplished by using either three-view thoracic radiographs or thoracic CT, although CT is much more sensitive than thoracic radiographs for detecting pulmonary metastases and is highly recommended for accurate staging,8 especially in patients requiring costly treatments or extensive surgical therapy.
MEDICAL ONCOLOGY PERSPECTIVE
Olya A. Smrkovski, DVM, DACVIM (oncology)
Metastatic disease is the principal cause of death in most dogs with oral malignant melanoma, especially those with good local tumor control.19 Chemotherapeutics with reported activity against oral malignant melanoma include carboplatin, cisplatin with piroxicam, dacarbazine, doxorubicin, and melphalan, but platinum drugs have the most activity.20-25 The overall response rate to carboplatin is 28%, and the reported response rate to a cisplatin-piroxicam combination is 18%.22,24

Olya A. Smrkovski, DVM, DACVIM (oncology)
Administration of adjuvant chemotherapy has failed to significantly improve overall survival in dogs with oral malignant melanoma.19-25 However, it continues to play a role in the management of this malignancy. At the University of Tennessee Veterinary Medical Center, chemotherapy is used in patients that develop progressive disease after the four initial treatments of the canine melanoma vaccine or when the vaccine is cost-prohibitive to owners. Metronomic (continuous, low-dose oral chemotherapy aimed at inhibiting tumor angiogenesis) is being increasingly used to treat solid tumors.26-28 It was not used in this case but can be considered in the future as more reports of its efficacy emerge.
Malignant melanoma is a highly immunogenic tumor. Of the multiple immunotherapeutic approaches investigated to date, Oncept, a xenogeneic DNA vaccine containing human tyrosinase (HuTyr) has shown the most promise. In one study, administration of this vaccine to 58 dogs with stage II and III oral malignant melanoma resulted in significant improvement in survival time compared with historical controls.29
The vaccine was licensed by the USDA in 2009 and is administered with a needle-free device every other week for a total of four treatments.30 Subsequent boosters at six-month intervals are recommended, provided there is no evidence of tumor progression.30 Oncept has not been associated with any systemic side effects; local side effects such as bruising and hematoma formation at the injection site are rare.29 Oncept is currently licensed to veterinary specialists boarded in either oncology or internal medicine.
RADIATION ONCOLOGY PERSPECTIVE
Nathan D. Lee, DVM, DACVR (radiation oncology)
Oral malignant melanomas that are incompletely excised or are too large for surgical resection can benefit from radiation therapy. Typically, oral malignant melanomas are treated with hypofractionated protocols or palliative radiation therapy because of the poor overall survival time associated with oral melanomas and reported low alpha-beta ratio of melanomas.31-35 Patients that have favorable prognostic factors (see below) and a reported longer median survival time may benefit more from a fractionated radiation protocol, which would decrease the chances of late side effects.

Nathan D. Lee, DVM, DACVR (radiation oncology)
The response rate of oral melanomas to radiation therapy is good; one report showed over an 80% response rate with, 51% of patients achieving a complete response to therapy.31 That same study, which is the largest study to date looking at dogs treated with external beam radiation therapy for oral melanomas, also showed that there was no difference in response rates or overall survival times among different radiation protocols, mainly because most patients die of metastatic disease.31
Three factors have been shown to effect median survival times in patients undergoing radiation treatments for oral melanomas:
1. The tumor's location (caudally located tumors have a poorer prognosis)
2. Bone lysis on imaging (those with bone lysis have a worse prognosis)
3. Tumor volume (dogs with macroscopic disease have a poorer prognosis).31
Patients that have none of these factors have a median survival time of 21 months. Patients with one of the factors have a median survival time of 11 months.31 The presence of two factors decreases the median survival time to five months, while the median survival time is only three months in patients with all three factors.31
Hypofractionated radiation therapy side effects are minimal. Most patients may experience mild mucositis around the tumor area, depending on tumor location and size. Partial to complete alopecia can develop in the radiation fields, especially in some breeds (e.g. poodles, Shih Tzus). The fur will grow back, but it can take up to six months and usually regrows in a different color. Late side effects to hypofractionated radiation therapy (occurring six months to several years after treatment) include osteonecrosis, muscle fibrosis, and secondary tumor formation. It is extremely rare to see late effects in patients with oral malignant melanoma because of the high metastatic rate of this disease, but if a patient did have favorable prognostic factors, late side effects may be more of a concern.
At the University of Tennessee we typically treat oral malignant melanomas with 8 Gy fractions spaced one week apart. We do not routinely use platinum-based chemotherapies as radiation sensitizers in these patients since there has been no significant difference in overall survival times reported.32,33 We do routinely recommend the melanoma vaccine be started immediately upon diagnosis and, anecdotally, have not seen any serious side effects associated with a combination of the melanoma vaccine and hypofractionated radiation therapy.
REFERENCES
1. Smith SH, Goldschmidt MH, McManus PM. A comparative review of melanocytic neoplasms. Vet Pathol 2002;39(6):651-678.
2. Goldschmidt MH. Benign and malignant melanocytic neoplasms of domestic animals. Am J Dermatopathol 1985;7(suppl):203-212.
3. Todoroff RJ, Brodey RS. Oral and pharyngeal neoplasia in the dog: a retrospective survey of 361 cases. J Am Vet Med Assoc 1979;175(6):567-571.
4. Wallace J, Matthiesen DT, Patnaik AK. Hemimaxillectomy for the treatment of oral tumors in 69 dogs. Vet Surg 1992;21(5):337-341.
5. Liptak JM, Withrow SJ. Cancer of the gastrointestinal tract. In: Withrow SJ, Vail DM, eds. Small animal clinical oncology. 4th ed. St. Louis, Mo: Saunders-Elsevier, 2007;455-475.
6. Williams LE, Packer RA. Association between lymph node size and metastasis in dogs with oral malignant melanoma: 100 cases (1987-2001). J Am Vet Med Assoc 2003;222(9):1234-1236.
7. Forrest LJ, Schwarz T. Oral cavity, mandible, maxilla and dental apparatus. In: Schwarz T, Saunders J, eds. Veterinary computed tomography. 1st ed. Ames, Iowa: Wiley-Blackwell, 2011;111-124.
8. Nemanic S, London CA, Wisner ER. Comparison of thoracic radiographs and single breath-hold helical CT for detection of pulmonary nodules in dogs with metastatic neoplasia. J Vet Intern Med 2006;20(3):508-515.
9. Raskin RE. Skin and subcutaneous tissues. In: Raskin RE, Meyer DJ, eds. Canine and feline cytology: a color atlas and interpretation guide. 2nd ed. St. Louis, Mo: Mosby Elsevier, 2010;1-64.
10. Bergman PJ, Kent MS, Farese JP. Melanoma. In: Withrow SJ, Vail DM, Page RL, eds. Small animal clinical oncology. 5th ed. St. Louis, Mo: Mosby Elsevier, 2013;321-334.
11. Esplin DG. Survival of dogs following surgical excision of histologically well-differentiated melanocytic neoplasms of the mucous membranes of the lips and oral cavity. Vet Pathol 2008;45(6):889-896.
12. Schwarz PD, Withrow SJ, Curtis CR, et al. Mandibular resection as a treatment of oral cancer in 81 dogs. J Am Anim Hosp Assoc 1991;27:601-610.
13. Schwarz PD, Withrow SJ, Curtis CR, et al. Partial maxillary resection as a treatment for oral cancer in 61 dogs. J Am Anim Hosp Assoc 1991;27:617-624.
14. Hedlund CS, Tangner CH, Elkins AD, et al. Temporary bilateral carotid artery occlusion during surgical exploration of the nasal cavity of the dog. Vet Surg 1983;12:83.
15. Berg J. Mandibulectomy and maxillectomy. In: Veterinary surgery: small animal. Vol. 2. St. Louis, Mo: Saunders, 2012.
16. Harvey CE. Oral surgery. Radical resection of maxillary and mandibular lesions. Vet Clin North Am Small Anim Pract 1986;16(5):983-993.
17. Matthiesen DT, Manfra Marretta S. Results and complications associated with partial mandibulectomy and maxillectomy techniques. Probl Vet Med 1990;2(1):248-275.
18. Fox LE, Geoghegan SL, Davis LH, et al. Owner satisfaction with partial mandibulectomy or maxillectomy for treatment of oral tumors in 27 dogs. J Am Anim Hosp Assoc 1997;33(1):25-31.
19. Thamm DH, Vail DM. Cancers of the gastrointestinal tract. In: Withrow SJ, Vail DM, eds. Withrow & MacEwen's small animal clinical oncology. 4th ed. St Louis, Mo: Saunders Elsevier, 2007;455-465.
20. Brockley LK, Cooper MA, Bennett PF. Malignant melanoma in 63 dogs (2001-2011): the effect of carboplatin chemotherapy on survival. N Z Vet J 2013;61(1):25-31.
21. Dank G, Rassnick KM, Sokolovsky Y, et al. Use of adjuvant carboplatin for treatment of dogs with oral malignant melanoma following surgical excision. Vet Comp Oncol 2012. [Epub ahead of print]
22. Boria PA, Murry DJ, Bennett PF, et al. Evaluation of cisplatin combined with piroxicam for the treatment of oral malignant melanoma and oral squamous cell carcinoma in dogs. J Am Vet Med Assoc 2004;224(3):388-394.
23. Page RL, Thrall DE, Dewhirst MW, et al. Phase I study of melphalan alone and melphalan plus whole body hyperthermia in dogs with malignant melanoma. Int J Hyperthermia 1991;7(4):559-566.
24. Rassnick KM, Ruslander DM, Cotter SM, et al. Use of carboplatin for treatment of dogs with malignant melanoma: 27 cases (1989-2000). J Am Vet Med Assoc 2001;218(9):1444-1448.
25. Ogilvie GK, Reynolds HA, Richardson RC, et al. Phase II evaluation of doxorubicin for treatment of various canine neoplasms. J Am Vet Med Assoc 1989;195(11):1580-1583.
26. Leach TN, Childress MO, Greene SN, et al. Prospective trial of metronomic chlorambucil chemotherapy in dogs with naturally occurring cancer. Vet Comp Oncol 2012;10(2):102-112.
27. Burton JH, Mitchell L, Thamm DH, et al. Low-dose cyclophosphamide selectively decreases regulatory T cells and inhibits angiogenesis in dogs with soft tissue sarcoma. J Vet Intern Med 2011;25(4):920-926.
28. Marchetti V, Giorgi M, Fioravanti A, et al. First-line metronomic chemotherapy in a metastatic model of spontaneous canine tumours: a pilot study. Invest New Drugs 2012;30(4):1725-1730.
29. Grosenbaugh DA, Leard AT, Bergman PJ, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res 2011;72(12):1631-1638.
30. USDA licenses DNA vaccine for treatment of melanoma in dogs. J Am Vet Med Assoc 2010;236(5):495.
31. Proulx DR, Ruslander DM, Dodge RK, et al. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Vet Radiol Ultrasound 2003:44(3):352-359.
32. Murphy S, Hayes AM, Blackwood L, et al. Oral malignant melanoma—the effect of coarse fractionation radiotherapy alone or with adjuvant carboplatin therapy. Vet Comp Oncol 2005:3(4):222-229.
33. Freeman KP, Hahn KA, Harris FD, et al. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987-1997). J Vet Intern Med 2003;17(1):96-101.
34. Overgaard J, Overgaard M, Hansen PV, et al. Some factors of importance in the radiation treatment of malignant melanoma. Radiother Oncol 1986;5(3):183-192.
35. Overgaard J. The role of radiotherapy in recurrent and metastatic malignant melanoma: a clinical radiobiological study. Int J Radiat Oncol Biol Phys 1986;12(6):867-872.