Definitively diagnosing hepatic vascular disease
When you have a patient with a hepatic vascular abnormality, how do you confirm it?
Hepatic vascular disease comprises a group of anomalies that result in abnormal blood flow either within or around the liver. The most common anomalies are congenital and acquired portosystemic shunts that form single or multiple direct connections between the portal vasculature and the systemic circulation. Clinicopathologic findings can be similar among the different vascular diseases, and more than one anomaly can occur within the same patient.

Getty/Flickr
Vascular imaging is the only method to definitively diagnose hepatic vascular disease. It is imperative that a specific diagnosis is made in patients with suspected hepatic vascular disease so appropriate therapy can be pursued.
DISEASE CLASSIFICATION
The consensus of the World Small Animal Veterinary Association Liver Standardization Group divides prehepatic vascular anomalies into three general categories1 :
1. Congenital intrahepatic and extrahepatic portosystemic shunts
2. Primary hypoplasia of the portal vein, which may or may not result in portal hypertension
3. Disorders of outflow.2
Most congenital intrahepatic and extrahepatic portosystemic shunts are a single vessel connecting the portal venous system to the systemic circulation. Several types of communication exist and have been described elsewhere.2,3
PORTOSYSTEMIC SHUNTS
Signalment
About 25% to 33% of portosystemic shunts are intrahepatic and occur most often in large-breed dogs.2 Predisposed breeds include Irish wolfhounds, Labrador retrievers, golden retrievers, and Australian cattle dogs. The remaining portosystemic shunts are extrahepatic and occur in small-breed dogs. Predisposed breeds include Yorkshire terriers, Maltese, Shih Tzus, and pugs.
Cats most often have extrahepatic portosystemic shunts, and domestic shorthaired, Persian, Himalayan, and Siamese cats are most commonly affected. A sex predilection toward males may exist in cats.
Clinical signs
The clinical signs observed with congenital portosystemic shunts depend on the degree of shunting and are primarily associated with the central nervous, gastrointestinal, and urinary systems.2 Ptyalism is a hallmark of portosystemic shunts in cats.4 Detailed explanations of the clinical signs observed with cases of portosystemic shunting can be found in internal medicine textbooks and review articles.2,4,5
PRIMARY HYPOPLASIA OF THE PORTAL VEIN
Primary hypoplasia of the portal vein may occur as a single anomaly or concurrently with a macroscopic portosystemic shunt. It is characterized by microscopic malformations of the hepatic vasculature.2 Primary hypoplasia of the portal vein without portal hypertension was previously called microvascular dysplasia, and Cairn and Yorkshire terrier breeds are overrepresented.
Primary hypoplasia of the portal vein with portal hypertension has been diagnosed in a variety of breeds, but Doberman pinschers are overrepresented.2,6 It is diagnosed when a patent but hypoplastic portal vein is present with a noncirrhotic liver. Other terms used in the literature include idiopathic noncirrhotic portal hypertension, hepatoportal fibrosis, and veno-occlusive disease. Clinical signs of primary hypoplasia of the portal vein are similar to those observed with portosystemic shunts, with the exception of ascites, which occurs only in cases of primary hypoplasia of the portal vein with portal hypertension. Acquired extrahepatic portosystemic shunts develop as a result of portal hypertension.
HEPATIC ARTERIOVENOUS MALFORMATION
Hepatic arteriovenous malformation is a rare vascular anomaly in which multiple aberrant communications occur among branches of the hepatic artery and portal vein. Hepatic arteriovenous malformation is usually a congenital disorder, has been diagnosed in dogs and cats, and was formerly called arteriovenous fistula. Abnormally high pressure results within the portal system, and a reversal of blood flow occurs, which is termed hepatofugal blood flow (blood flow away from the liver).7 The resultant portal hypertension leads to acquired extrahepatic portosystemic shunts. Clinical signs are similar to those of portosystemic shunts, and ascites is observed in 75% of cases.2
LABORATORY TESTING
Hematologic abnormalities often reveal evidence of marked hepatic dysfunction. Twelve-hour fasting preprandial and two-hour postprandial serum bile acid concentration tests are preferred for detecting liver dysfunction in patients suspected of having portosystemic vascular anomalies.2 Samples are easy to obtain because serum harvested from whole blood samples is all that is required. The sensitivity for preprandial and postprandial serum bile acid concentration tests is 100% for detecting hepatobiliary disease.8
Ammonia tolerance tests and fasting or postprandial blood ammonia concentration tests are less commonly used because samples must be processed within 20 minutes of the blood draw.2 In fact, Antech Diagnostics does not process ammonia concentrations because of the instability of the samples.9 Ammonia tolerance tests also require that 100 mg/kg of ammonium chloride be given to patients either orally or rectally.
The use of fasting ammonia concentrations and fasting serum bile acid concentrations in identifying portosystemic shunts was recently compared, and the sensitivity and specificity were 98% and 89.1% for fasting ammonia and 88.1% and 17.9% for fasting serum bile acids.10 A second study evaluated only postprandial ammonia concentrations as a diagnostic test for detecting hepatic disease. The sensitivity and specificity for detecting portosystemic vascular anomalies in dogs increased to 100% and 91% six hours postprandially.11 Elevated results in any of the liver function tests indicate hepatic insufficiency but are not specific for hepatic vascular disease.
THE ROLE OF DIAGNOSTIC IMAGING
Diagnostic imaging is the only method to specifically diagnose hepatic vascular disease. The goals of imaging are to confirm a vascular anomaly, quantify the degree of shunting, and characterize the morphology and location of the anomaly. The information that diagnostic imaging can provide is critical to the development of a therapeutic plan, including surgical planning when applicable.
Differentiating between intrahepatic and extrahepatic portosystemic shunts depends on where the shunt is found to diverge from the portal vein. If the shunting vessel diverges cranial to T13, then it is usually an intrahepatic portosystemic shunt. If it is caudal to T13, it is usually an extrahepatic portosystemic shunt.12 Prognosis is also variable depending on the type of vascular disease and severity of shunting.
Available techniques include portovenography, ultrasonography, scintigraphy, computed tomography (CT), and magnetic resonance imaging (MRI) (Table 1). Each imaging modality varies in its sensitivity and specificity, cost, and availability.

Table 1 Advantages and Disadvantages of Available Techniques for Imaging Hepatic Vasculature
PORTOVENOGRAPHY
Portovenography is the gold standard diagnostic imaging modality to anatomically diagnose congenital or acquired portosystemic shunts.13
Operative portograms
Intraoperative techniques that have been described include mesenteric portography and transsplenic portovenography.
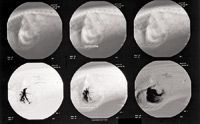
Figure 1. A normal operative mesenteric portogram. These fluoroscopic images, from left to right, depict the flow of contrast injected through a jejunal vein into the portal vein. The bottom three images are digitally subtracted to increase the contrast between the vessels and surrounding tissue. Note the normal arborizing appearance of the portal branches within the liver. (Figures 1 & 2 courtesy of Philip Steyn, BVSc, MS, DACVR, director of professional services and chief radiologist for Antech Imaging Services.)
Mesenteric portography is performed through surgical catheterization of a jejunal vein, injection of 2 ml/kg of a nonionic water-soluble contrast medium such as iohexol, and visualization of the portal venous system by fluoroscopy.14,15 An example of a normal operative mesenteric portogram is shown in Figure 1; an abnormal one is shown in Figure 2.
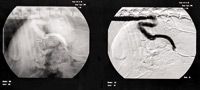
Figure 2. An operative mesenteric portogram of an extrahepatic portosystemic shunt. By using fluoroscopy, the flow of contrast is noted entering the azygous vein through a shunt vessel that unites the portal vein and azygous vein. The image on the right is digitally subtracted to enhance contrast between the vessels and surrounding tissue. Note the absence of flow through the liver because of the shunt.
Transsplenic portovenography involves surgical catheterization of a splenic venule followed by injection of contrast.16,17
With either technique, the caudal vena cava or azygous vein opacifies before or at the same time as the liver when shunting is present. The normal arborizing appearance of the intrahepatic portal vasculature is attenuated or absent and may remain so after corrective ligation in cases of multiple macroscopic portosystemic shunts or primary hypoplasia of the portal vein.7
Operative portovenography of a jejunal vein or splenic vein is invasive but provides excellent imaging of shunting vessels' morphology and location. The sensitivity of operative portovenography is between 85% and 100%.2 Disadvantages include the requirement of mobile fluoroscopy equipment and laparotomy; however, laparotomy is indicated regardless for surgical treatment of portosystemic shunts.
Less-invasive methods
Methods of visualizing the portal venous system that do not require laparotomy include percutaneous splenoportography and cranial mesenteric arterial portography.
Percutaneous splenoportography is accomplished through ultrasound-guided injection of 2 to 4 ml/kg of a nonionic water-soluble contrast medium into the splenic parenchyma.2 Fluoroscopy is used to visualize venous drainage from the spleen, followed by systemic venous flow in the presence of portosystemic shunts. One drawback of splenoportography is that mesenteric-systemic shunts located caudal to the splenic vein are not opacified.12,17 Splenoportography has been extensively investigated and reviewed, but its sensitivity and specificity have not been reported.12,17
Cranial mesenteric arterial portography is accomplished through catheterization of a femoral artery and injection of 1.5 to 3 ml/kg of nonionic water-soluble contrast medium.12 The contrast medium opacifies the portal vein, following circulation through the intestinal vasculature.
ULTRASONOGRAPHY
Ultrasonography is a noninvasive method of reliably diagnosing portosystemic shunts and is the imaging modality most widely used to diagnose portosystemic shunts. It does not require general anesthesia and allows visualization of the entire abdomen so that concurrent abnormalities may be detected. The accuracy of ultrasonography, however, largely depends on the operator's skill and experience.
Evaluating portosystemic shunts
Ultrasonography allows direct visualization of shunting vessels, differentiation between intrahepatic and extrahepatic portosystemic shunts, and categorization of the subtype of portosystemic shunts. The use of Doppler color-flow ultrasonography allows detection of turbulent blood flow and measurement of portal blood flow velocity. This method has enhanced the sensitivity of ultrasonography in diagnosing portosystemic shunts in recent reports.18
A reduced ratio of portal vein diameter to aortic diameter (PV:Ao), as measured by ultrasonography, has been shown to predict the presence of extrahepatic portosystemic shunts in one recent study.19 A right intercostal approach, usually between the 11th and 12th and ribs, is used to visualized both the portal vein and aorta in cross-section. A PV:Ao ≤ 0.65 is predictive of an extrahepatic portosystemic shunt. A PV:Ao ≥ 0.8 excludes the presence of an extrahepatic portosystemic shunt.19 A similar right intercostal approach is used to visualize and compare the portal vein and caudal vena cava. A PV:CVC of ≥ 0.75 has been shown to exclude the presence of an extrahepatic portosystemic shunt.19 Ultrasound may also be used to scan the portal vein for aberrant connections.18,19
Intrahepatic portosystemic shunts are generally easier to visualize (Figure 3) than extrahepatic portosystemic shunts are because of interference by the gastrointestinal tract with visualization of extrahepatic portosystemic shunts. A method of ultrasound-guided percutaneous splenic injection of agitated saline solution and heparinized blood has been described to identify portosystemic shunting through visualization of microbubbles in the portal vein, caudal vena cava, and right atrium in different patterns.20 The sensitivity and specificity have not yet been determined for this method.
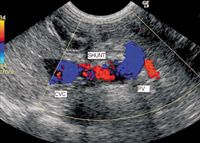
Figure 3. A Doppler ultrasonogram of a dog with an intrahepatic portosystemic shunt. This image depicts a shunting vessel within the right lateral liver lobe, connecting the portal vein and caudal vena cava. (Figure 3 courtesy of Philip Steyn, BVSc, MS, DACVR, director of professional services and chief radiologist for Antech Imaging Services.)
Additional ultrasonographic findings that may be detected in cases of portosystemic shunts include microhepatica, decreased numbers of hepatic vessels, renomegaly, and urolithiasis.18 The sensitivity and specificity for detection of intrahepatic portosystemic shunts is 95% to 100% and 100%, respectively, compared with the more variable sensitivity and specificity values reported for extrahepatic portosystemic shunts of 81% to 90% and 67% to 97%, respectively.3,18,19 The overall sensitivity and specificity for the ultrasonographic diagnosis of all types of portosystemic shunts is 92% to 95% and 98% as reported in recent studies.18,19
Evaluating other hepatic disorders
Acquired extrahepatic portosystemic shunts often occur secondary to portal hypertension, as may be created by conditions such as primary hypoplasia of the portal vein, hepatic arteriovenous malformation, and hepatic cirrhosis. Shunt vessels are generally small, multiple, and visualized most commonly near the kidneys or spleen.3,21
Hepatic arteriovenous malformation is diagnosed through ultrasonography by detecting an enlarged portal vein, dilated and tortuous intrahepatic portal branches, and hepatofugal flow.21 Portal blood flow is normally nonpulsatile, but in cases of hepatic arteriovenous malformation, Doppler pulse wave examination reveals a pulsatile pattern within the portal venous system similar to that within the hepatic artery.22
NUCLEAR SCINTIGRAPHY
Portal scintigraphy is a noninvasive method of diagnosing portosystemic shunts by using radioisotopes to image portal blood flow. Patients with primary hypoplasia of the portal vein can have normal or abnormal scans, but calculated shunt fractions are usually much lower in these patients than in patients with macroscopic portosystemic shunts. Nuclear portal scintigraphy is not a described imaging modality to diagnose hepatic arteriovenous malformation.
Per-rectum portal scintigraphy
Per-rectum portal scintigraphy (PRPS) is performed by administering 10 to 20 millicuries (mCi) of 99mTc-pertechnetate through the distal colon. The radioisotope is absorbed by the colonic veins, followed by the caudal mesenteric vein, portal vein, liver, and, finally, heart (Figure 4). In the presence of a portosystemic shunt, the radioisotope bypasses the liver to reach the heart first (Figure 5).
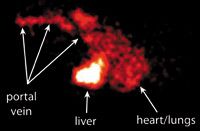
Figure 4. A normal per-rectum portal scintigram using technetium 99mTc-pertechnetate in a dog. The flow of the radionucleotide is depicted as it is absorbed into the portal vein, perfuses the liver, and then reaches the heart. (Figures 4 & 5 courtesy of Michael Broome, DVM, MS, DABVP, and Rachel Moon, DVM, at Advanced Veterinary Medical Imaging.)
Patients are sedated and placed in right lateral recumbency for PRPS. The locations of the heart and liver are marked as regions of interest, and a gamma camera collects images over two or three minutes. Specialized software is used to measure shunt fraction. Shunt fractions > 15% are considered positive for portosystemic shunts, and a mean shunt fraction of 84% was found in patients with portosystemic shunts in one study using PRPS.23
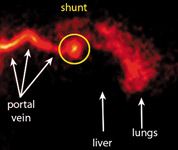
Figure 5. A portal scintigram from a dog with an extrahepatic portosystemic shunt. Note how the radionucleotide bypasses the liver and is seen highlighting the heart and lungs.
PRPS is simple to perform, noninvasive, and yields rapid quantitative results. Distinct disadvantages include the need for special certification and radiation safety measures. PRPS does not provide detailed images of the shunting vessels and cannot distinguish between single congenital portosystemic shunts and multiple acquired portosystemic shunts. A false positive study may occur if the radioisotope is absorbed into the systemic circulation through the caudal rectal vein. Portal streamlining, which is the nonuniform distribution of blood (or radioisotope) to the liver due to preferential delivery by discrete portal channels, is a recognized cause of falsely interpreted studies.24 The nonuniform appearance of radioactivity within the liver is a normal physiologic phenomenon.
Transsplenic portal scintigraphy
Transsplenic portal scintigraphy was developed to overcome the poor absorption rate and lack of anatomic detail of PRPS. Ultrasound-guided injection of 1 or 2 mCi of 99mTc-pertechnetate into the splenic parenchyma results in absorption by the splenic vein, followed by the left gastric vein and main portal vein.25 Scans are evaluated similar to PRPS.
A nuclear portovenogram is imaged in most transsplenic portal scintigraphy studies because of the greater absorption and density of radioisotope, which allows for identification of shunt number and termination.26 Another advantage over PRPS is the decreased dose of radioisotope required. Disadvantages of transsplenic portal scintigraphy include risk of splenic hemorrhage, intraperitoneal injection resulting in a nondiagnostic scan, and the possibility of missing a portosystemic shunt caudal to the splenic vein.25
Comparison of the two methods
A recent study comparing PRPS and transsplenic portal scintigraphy concluded that both methods are 100% sensitive for detection of macroscopic shunts in dogs with portosystemic shunts. The reported specificity from the study was 100% for transsplenic portal scintigraphy and 95% for PRPS. It also concluded that transsplenic portal scintigraphy provides significantly better scan quality than PRPS does and has significantly improved ability to delineate shunt anatomy.26
CT ANGIOGRAPHY
CT angiography is the gold standard for evaluating hepatic vascular anomalies in people and is being used to diagnose portosystemic shunts in animals with increasing frequency. Methods of performing helical CT angiography to image portosystemic shunts in dogs have been recently described.27,28 Single-phase CT angiography images only the portal venous phase of hepatic blood flow, while dual-phase images both the arterial and portal phases.
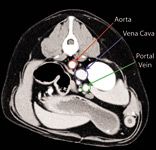
Figure 6. Dual-phase CT angiography. This axial CT image is of a normal dog. Note the contrast enhancement of the vasculature. (Figures 6-11 courtesy of Michael Broome, DVM, MS, DABVP, and Rachel Moon, DVM, at Advanced Veterinary Medical Imaging.)
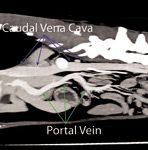
Figure 7. Dual-phase CT angiography. This sagittal view of the same dog as in Figure 6 is a multiplanar reformat created from the raw helical data file and built-in software in the helical CT scanner (HiSpeed NX/i dual-detector helical CT unit-GE Healthcare) used for the study. The dogߣs head is to the left.
Procedure
Patients must be anesthetized for CT. Before image acquisition, patients are hyperventilated to induce apnea, which reduces respiratory motion artifact in the images. The start time for image acquisition is determined by an initial test intravenous bolus of 0.55 ml/kg of iodinated contrast medium and scanning for the time of maximum opacification of either the hepatic artery or portal vein. Images are then acquired after an injection of 2.2 ml/kg of iodinated contrast medium, while scanning the entire abdomen from caudal to cranial for the arterial phase and cranial to caudal for the portal phase (Figures 6 & 7).
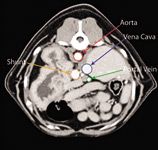
Figure 8. Dual-phase CT angiography of an extrahepatic portosystemic shunt. The axial CT image reveals contrast enhancement within a shunt vessel.
Usefulness
CT angiography has consistently diagnosed hepatic vascular anomalies, including intrahepatic and extrahepatic portosystemic shunts and hepatic arteriovenous malformation, in recent reports.27-31 The origin, course, and insertion of extrahepatic and intrahepatic portosystemic shunts can be accurately detailed (Figures 8 & 9). This method is ideal for diagnosing dorsally located shunts that are difficult to image with ultrasonography, such as left gastric vein shunts and portoazygous shunts.29,31
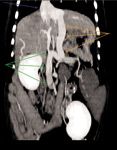
Figure 9. Dual-phase CT angiography of an extrahepatic portosystemic shunt. The image is a sagittal view of the dog in Figure 8 and a multiplanar reformat created from the raw helical data file. The shunt vessel (orange arrows) can be seen connecting the portal vein (green arrows) to the caudal vena cava (purple arrow). Note the hypoplastic portal branches. The dogâs right is to the left.
CT can also aid in the diagnosis of multiple acquired portosystemic shunts, which are difficult to detect with ultrasonography and cannot be distinguished from single macroscopic shunting through nuclear portal scintigraphy (Figures 10 & 11). CT is also especially useful in the diagnosis of hepatic arteriovenous malformation when a dual-phase scan is performed. Opacification of the portal veins is seen concurrent with the arterial phase of the scan in cases of hepatic arteriovenous malformation.30
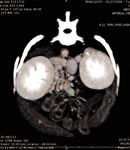
Figure 10. Dual-phase CT angiography of multiple acquired shunts. The orange circle is encompassing multiple acquired shunts between the portal vein and caudal vena cava at the level of the left kidney.
The primary disadvantages of CT compared with ultrasonography are the requirement of general anesthesia and reduced availability of equipment. Cost may also be a limiting factor. Patient motion and poor resolution in small patients are additional limits to CT. The information obtained from CT angiography is the same as provided by operative mesenteric portography.28 However, preoperative CT also allows surgical planning, which can reduce anesthetic time and tissue manipulation. At this time, sensitivity and specificity values are not established for CT diagnosis of hepatic vascular anomalies.
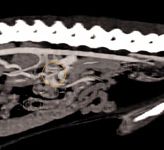
Figure 11. Dual-phase CT angiography of multiple acquired shunts. The image is a sagittal view of the dog in Figure 10.
MAGNETIC RESONANCE IMAGING
The use of MRI to diagnose hepatic vascular anomalies is not established in veterinary medicine. Magnetic resonance angiography (MRA) has been described as a noninvasive technique for imaging portal vasculature.32,33
One study reports 80% sensitivity and 100% specificity for the diagnosis of any shunt type, and 63% sensitivity and 97% specificity for the diagnosis of multiple extrahepatic portosystemic shunts.32 Smaller vessels may not emit a detectable signal, making it difficult to diagnose multiple acquired shunts or some smaller single extrahepatic portosystemic shunt by using MRA.32 Figure 12 depicts a portoazygous shunt detected by MRA.
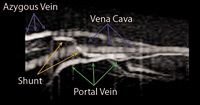
Figure 12. MRA of an extrahepatic portosystemic shunt. This sagittal MRA image of a dog with a single extrahepatic portosystemic shunt shows contrast enhancement of a portoazygous shunt. The dogâs head is to the left. (Figure 12 courtesy of the Department of Veterinary Clinical Sciences at Washington State University.)
Image acquisition times are longer for MRI than for CT, resulting in lengthened anesthetic periods for patients undergoing MRI. A more recent study on contrast-enhanced MRA used nonselective angiography, which reduced acquisition time but made interpretation more complicated by enhancing all abdominal vasculature.33 To date, no advantage to the use of MRA vs. CT angiography has been established.
CONCLUSION
A variety of diagnostic imaging modalities have been described to evaluate hepatic vascular disease. The ideal method will provide a high degree of sensitivity and specificity and fine resolution for detailed anatomic reconstruction and be both affordable and available while presenting minimal risk to patients. Our ability to characterize hepatic vascular diseases, obtain early diagnoses, formulate therapeutic plans, and provide prognostic information will expand as advanced diagnostic imaging techniques are applied to these diseases with greater frequency.
To view the references for this article, visit dvm360.com/HepaticVascularRefs.
Valerie L. Case, DVM, DACVIM
Center for Veterinary Specialty Care
1712 Frankford Road, Suite 108
Carrollton, TX 75007
Brian C. Norman, DVM, DACVIM
Veterinary Specialists of the Valley
22123 Ventura Blvd.
Woodland Hills, CA 91364
Jason Francis, DVM, DACVR
Ventura Veterinary Imaging Specialists
4221 E. Main St.
Ventura, CA 93003