Degenerative lumbosacral stenosis in dogs
Degenerative lumbosacral stenosis is a common cause of cauda equina syndrome and a relatively frequent neurologic disorder in older dogs. If this condition is recognized early, treatment may help alleviate significant morbidity.
Degenerative lumbosacral stenosis is a common cause of cauda equina syndrome and a relatively frequent neurologic disorder in older dogs. If this condition is recognized early, treatment may help alleviate significant morbidity.
The terminology applied to clinical syndromes that affect the spinal cord segments, the spinal nerve roots, and the spinal nerves that contribute to the sciatic nerve innervation of the perineum, urinary bladder, urethral and anal sphincters, and tail can be confusing. Specifically, diseases that affect these structures can result in varying degrees of pelvic limb paresis and urinary and fecal incontinence because they are components of the seventh lumbar (L7) spinal nerve, the first through the third sacral (S1-S3) spinal nerves, and the caudal nerves.
The term lumbosacral disease encompasses any pathologic condition that affects the region of the spinal cord segments that contribute to the sciatic nerve and sacral and caudal nerves. Cauda equina syndrome implies pathologic conditions that affect the last several pairs of spinal nerve roots. Thus, lumbosacral disease and cauda equina syndrome are not specific conditions. Instead, they are the clinical manifestations of a variety of diseases that result in dysfunction of either the lumbosacral spinal cord segments or spinal nerve roots, respectively. Consequently, these two syndromes can result in similar clinical signs.
Causes of lumbosacral disease or cauda equina syndrome include degenerative lumbosacral stenosis; congenital lumbosacral stenosis; acute intervertebral disk extrusion; diskospondylitis; traumatic fractures, luxation, or subluxation; primary neoplasia (involving the vertebral body such as osteosarcoma, hemangiosarcoma, and fibrosarcoma or neural structures such as peripheral nerve sheath tumors or meningioma); metastatic neoplasia such as prostatic carcinoma; inflammatory disease such as neuritis of the cauda equina; spinal empyema; and congenital lesions of the vertebrae.1-3 While not specifically included under the terms lumbosacral disease or cauda equina syndrome, other conditions that can cause similar clinical signs are fibrocartilaginous embolic myelopathy, myopathies, myasthenia gravis, thrombosis of the distal aorta and iliac arteries, and orthopedic disease of the coxofemoral and stifle joints, such as hip dysplasia, cruciate ligament disease, and polyarthropathies.
A detailed discussion of all these causes is beyond the scope of this article. Instead, this article concentrates on the anatomical structures, clinical signs, diagnostic evaluation, therapeutic interventions, and prognosis involved in degenerative lumbosacral stenosis.
ANATOMICAL STRUCTURES
To understand the neurologic signs and various diseases that affect the lumbosacral region of the nervous system, a strong knowledge of the anatomy is needed.
Neural structures
The L4-S1 spinal cord segments are known as the lumbosacral intumescence since the spinal cord is grossly enlarged in this area because of the large collection of neuronal cell bodies in the gray matter that contribute to pelvic limb innervation. As the spinal cord ends, it tapers. This anatomical tapering is called the conus medullaris. 4 In dogs, the spinal cord terminates within the vertebral canal at about the level of the L6 vertebra, depending on the dog's size.4 In large dogs, the end of the spinal cord tends to be more cranial than in smaller breeds.5 Consequently, the cell bodies that contribute to the L7 spinal nerve are approximately located within the spinal canal at L4-L5 vertebrae.6 The cell bodies for the sacral and caudal nerve roots are located slightly more caudally. This disparity between the end of the spinal cord and the vertebral canal is the result of the differential growth patterns of these two structures, with the spinal cord ceasing development before the completion of vertebral column growth.4
Even though the spinal cord ends, the dura mater continues caudally as a dural sac, known as the lumbar cistern. 4 In dogs, the dural sac continues caudally for a variable distance, occasionally within the sacral vertebral canal. The ventral (motor) and dorsal (sensory) nerve roots exit and enter the spinal cord, respectively, within the dura mater as intradural nerve roots. Although they are known as extradural spinal nerve roots, they remain covered by meninges as they traverse the spinal canal to exit their respective intervertebral foramina.
At the level of the intervertebral foramen, the spinal nerve roots join and become a spinal nerve. There are seven lumbar spinal nerves. The individual lumbar spinal nerves exit through the intervertebral foramen caudal to the vertebra of the corresponding number. For example, the L6 spinal nerve exits through the intervertebral foramen formed by the L6 and L7 vertebrae, while the L7 spinal nerve exits through the intervertebral foramen formed by the L7 vertebra and the sacrum. Spinal nerve roots that originate more cranially will lie more laterally within the vertebral canal. Therefore, L7 spinal nerve roots are located lateral to the S1 spinal nerve roots, which are lateral to S2 spinal nerve roots, and so forth. Once outside the vertebral canal, the L6, L7, and S1 spinal nerves (with occasional contributions from the L5 and S2 spinal nerves) converge to become the sciatic nerve.4,7 The S1-S3 spinal nerves come together to form the pelvic and pudendal nerves, while the caudal nerves innervate the tail.4
Bony, cartilaginous, ligamentous, and vascular structures
In addition to the neural structures, a strong understanding of the bony, cartilaginous, ligamentous, and vascular structures that make up the vertebral column from the L7 vertebra through the sacrum is important. The vertebral canal in the L7 vertebra and sacrum tends to be dorsoventrally flattened compared with elsewhere in the vertebral column. Lateral depressions in the vertebral canal within the L7 body form lateral recesses for the L7 spinal nerve to exit the intervertebral foramen just cranial to the L7-S1 intervertebral disk (Figure 1). At the level of the intervertebral disk, the vertebral canal is composed of aspects of the L7 vertebra, intervertebral disk, interarcuate ligament, and sacrum. The dorsal portion of the vertebral canal is composed of the dorsal lamina of the L7 vertebra and the sacrum as well as the interarcuate ligament that spans the space between the vertebrae. The ventral portion of the canal is composed of the body of the L7 vertebra, the dorsal anulus fibrosus of the intervertebral disk, and the body of the sacrum. Overlying these structures is the dorsal longitudinal ligament. The articulations between the articular processes and facets of the L7 vertebra and the sacrum form synovial joints that dorsolaterally overlie the vertebral canal.
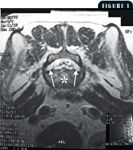
1. A T2-weighted magnetic resonance image of the caudal aspect of the L7 vertebra in the axial plane. Note the lateral recesses (arrows). The asterisk is overlying the body of the L7 vertebra.
The vascular structures also contribute to the pathophysiology of degenerative lumbosacral stenosis. The internal vertebral venous plexus (often referred to as the vertebral venous sinus) lies on the ventral aspect of the vertebral canal. This paired venous structure winds its way down the entire vertebral canal, diverging laterally at the intervertebral disk and converging within the body of the vertebra, often interconnecting at the convergence. In addition to the spinal nerves, spinal arteries and veins traverse the intervertebral foramen. The intervertebral foramen is more akin to a canal.8 It is formed by the caudal vertebral notch of the L7 vertebra and the cranial notch of the sacrum (cranially and caudally), the intervening intervertebral disk (ventrally), and the articular processes and synovial joint capsules (dorsally).
PATHOLOGIC FINDINGS
Degenerative lumbosacral stenosis involves varying degrees of pathologic changes of several anatomical structures. Abnormalities include
- Chronic Hansen's Type II degenerative intervertebral disk disease of the L7-S1 intervertebral disk
- Hypertrophy and ventral folding of the interarcuate ligament
- Osteoarthritis and subsequent joint capsule proliferation of the articular facets of L7-S1 articulation
- Subluxation of the sacrum in relation to the L7 vertebra
Occasionally, osteochondrosis of the cranial sacral or caudal L7 end plate may be seen.9,10
In normal dogs, motion of the vertebral column in flexion and extension progressively increases caudally in the lumbar vertebral column, with the L7-S1 articulation demonstrating the greatest amount of mobility.11 Instability of the L7-sacral articulation has been identified in dogs with degenerative lumbosacral stenosis.2,12 Other studies have found reduced mobility of the lumbosacral junction.13 Objective radiographic evidence of instability has also been identified.13,14 Often the caudal articular processes and facets of the L7 vertebra are displaced caudally in relation to the sacrum. As a result, the L7-S1 intervertebral foramen is narrowed, compressing the L7 spinal nerve. This may lead to the chronic pain. Compression at the cauda equina can also be exacerbated with extension of the pelvic limbs.13,14
A surgical technique for internal fixation of the L7-S1 articulation has been defined.15 Often this technique is combined with decompressive surgery (see below). Some neurologists have advocated internal fixation for all dogs with degenerative lumbosacral stenosis.16 A direct comparison between the outcome of dogs treated by decompression alone vs. decompression and stabilization has not been made. Consequently, despite evidence of instability in some dogs with degenerative lumbosacral stenosis, the role of instability at the L7-sacral articulation and its effect on the pathophysiology of degenerative lumbosacral stenosis remains controversial.
Vascular compromise may also play a role in the pathophysiology of degenerative lumbosacral stenosis. In people, intermittent claudication, numbness, pain, and weakness of the legs with exercise that resolves with rest is thought to be the result of nerve root ischemia from compression of radicular arteries.17 Experimentally, compression within the vertebral canal of the cauda equina or within the L7-sacral foramen of the L7 spinal nerve results in vascular compromise, leading to ischemia of the L7 dorsal root ganglia.17 Although not documented, ischemia may play a role in clinical cases, given the similarity between clinical signs of degenerative lumbosacral stenosis and intermittent claudication in people.2
SIGNALMENT
Affected dogs are often large-breed older dogs, with German shepherds being overrepresented.8,12,18-20 Other more commonly affected breeds include Great Danes, Airedale terriers, Irish setters, English springer spaniels, boxers, and Labrador and golden retrievers.19 Affected dogs are usually between 6 and 7 years of age at presentation.12,18 There is also a male predisposition.10,12,20,21
HISTORY AND CLINICAL SIGNS
In general, affected dogs have a long-term history of pelvic limb weakness that may be intermittent. Typically, dogs progressively worsen over time.
Clinical signs are referable to dysfunction of the L7, S1-S3, or caudal spinal nerve roots. Abnormalities in any one part of the lower motor neuron unit, the cell body within the spinal cord, spinal nerve roots, peripheral nerves, neuromuscular junction, or muscle, are clinically indistinguishable from a lesion in another part of the lower motor neuron unit.22 So be sure to consider the entire lower motor neuron unit when evaluating patients with signs consistent with degenerative lumbosacral stenosis.
The most common clinical sign associated with degenerative lumbosacral stenosis is pain at the lumbosacral articulation.8,12,18,21,23 Other clinical signs may be present in various combinations depending on severity and which nerve roots are affected. In general, varying degrees of paraparesis may also be present. Patients may assume a crouched stance with overflexion of the hip, stifle, and hock joints. Occasionally, dogs may hold a pelvic limb off the ground; this is known as a root signature.
Gait analysis reveals a shortened, choppy stride. Occasionally, the paresis results in knuckling over and subsequently ambulating on the dorsum of the paw. In extreme cases, the severity of the paresis is great enough to give the impression of proprioceptive ataxia. However, true incoordination, or ataxia, is usually not seen. Affected dogs often have difficulty rising, jumping, and climbing stairs. Exercise intolerance is sometimes noted.
Urinary incontinence, ranging from occasional overflow of urine during sleep to constant dribbling of urine, can be observed. Urethral sphincter dyssynergia can also be occasionally seen. In this case, the patient can initiate urination, but voiding ceases abruptly and prematurely with continued straining. Likewise, defecation can be affected. Abnormalities include an inability to posture or a change in posture, straining to defecate, and fecal incontinence.
Lastly, there can be paresis of the tail as demonstrated by an inability to wag the tail or raise the tail during urination or defecation. Occasionally, affected dogs self-mutilate their tails.2
NEUROLOGIC EXAMINATION
Neurologic examination findings range from pain as the sole finding to severe motor and sensory deficits. As mentioned previously, the pelvic limb gait may be shortened and choppy with a plantigrade stance. Often the gait has characteristics similar to those of lameness from orthopedic disease. Postural reaction deficits can be observed with hopping, hemiwalking, or, in smaller patients, extensor postural thrust. Proprioceptive positioning (often referred to as conscious proprioception) may be delayed. The withdrawal reflex can be reduced. Likewise, the cranial tibial and gastrocnemius reflexes may be reduced or absent, though these reflexes are not reliably detected in normal dogs.6 However, the patellar reflex is normal. Occasionally, the patellar reflex may be brisk, which is known as pseudohyperreflexia. 18 The exaggerated reaction is the result of a lack of tone from the caudal thigh muscles rather than a lack of inhibition by the descending upper motor neurons as seen in spinal cord lesions cranial to the L4 spinal cord segment.
With passive range of motion of the limb, you may note hypotonia, a quality of the flaccid paresis or paralysis seen with lower motor neuron disease. Similarly, decreased tail tone may be apparent. On palpation, you may detect atrophy of the semimembranosus, semitendinosus, gastrocnemius, and cranial tibial muscles, which are innervated by the sciatic nerve and its two main branches, the tibial and peroneal nerves. The perineal reflex may be reduced or absent.
Evaluation of the anus may reveal a dilated anal sphincter. Digital rectal examination may uncover decreased tone of the external anal sphincter. Simultaneous rectal examination and evaluation of the perineal reflex elicited by palpating the prepuce (innervated by a branch of the femoral nerve) or penis or vulva (innervated by a branch of the pudendal nerve) may reveal a decrease in strength of contraction of the external anal sphincter. Additionally, you may elicit a painful response during digital rectal palpation if you apply pressure to the ventral L7-S1 articulation.
Palpation of the bladder may reveal a large bladder that is easily expressed manually. Alternatively, you may note constant dribbling of urine. In female dogs, the perineum may also be wet with urine. You may also detect a painful response when applying pressure over the lumbosacral articulation. When palpating over the lumbosacral joint, take care to differentiate whether a painful response is related to lumbosacral disease or due to an orthopedic condition. Occasionally, positioning the patient in lateral recumbency during palpation can help eliminate pressure transmitted across the joints of the pelvic limbs. A test often used to detect a painful response to manipulation of the lumbosacral joint is extension of the coxofemoral joints. Extending the coxofemoral joints extends the lumbosacral joint, which may elicit a painful response; however, it is often difficult to distinguish painful coxofemoral disease from lumbosacral pain.
DIAGNOSTIC EVALUATION
Clinicopathologic testing
Disease affecting the lumbosacral articulation rarely results in metabolic derangement. While hematologic and biochemical tests rarely contribute to the diagnosis, evaluation is warranted because patients occasionally have concurrent illnesses. Urinalysis may reveal evidence of bacterial cystitis secondary to urethral sphincter incompetence. Perform a bacteriologic culture in patients with evidence of bacterial cystitis.
Electrophysiologic testing
Electrophysiologic testing can aid in the diagnosis of lumbosacral disease, but it rarely helps identify a definitive cause. Instead, it supports clinical suspicion of lumbosacral disease. Additionally, electrophysiologic evaluation of the thoracic limbs may uncover a more generalized lower motor neuron disease, thereby eliminating lumbosacral disease from further consideration.
Electrophysiologic tests should include electromyography and nerve stimulation testing. Evaluating sensory nerve conduction and the F wave may provide additional information, but their usefulness needs further investigation. In one report of 13 dogs, electromyogram abnormalities were identified only in dogs with compression seen at surgery, and no abnormalities were identified in dogs lacking compression.24 Furthermore, five of six dogs studied had normal motor nerve conduction velocity of the sciatic nerves but reduced amplitude and polyphasic dispersion of the compound muscle action potential indicative of axonal damage.24
Imaging
Without a doubt, a diagnosis of degenerative lumbosacral stenosis relies heavily on imaging procedures. The most commonly used imaging techniques include plain radiography, myelography, epidurography, computed tomography (CT), and magnetic resonance imaging (MRI).
Plain radiography
Plain radiography is frequently used as a screening test because it is readily accessible. However, radiographs of the lumbosacral articulation are often difficult to interpret because of the complexity of the anatomy and overlying wings of the ilium bilaterally. Furthermore, soft tissue structures, often the main structures involved in degenerative lumbosacral stenosis, are not seen with plain radiography. As a result, false positive and false negative diagnoses are often reached based on radiographs alone.25
The real value of radiography lies in the ability to exclude other causes from consideration. Diseases such as diskospondylitis and neoplasia and traumatic injury are often easily identified radiographically. Plain radiography is also useful in identifying anatomical anomalies of the vertebrae such as transitional vertebrae, or vertebrae with anatomical features characteristic of the two different regions of the vertebral column (Figures 2 & 3). In one study, dogs with a combination of degenerative changes and transitional vertebrae at the lumbosacral articulation were significantly more likely to have degenerative lumbosacral stenosis then dogs with degenerative changes or transitional vertebrae alone.26

Figure 2: A lateral lumbosacral radiograph of a dog with a transitional vertebra. Note the sacralization of the L7 vertebra (arrow). The craniocaudal length of the L7 vertebral body is shortened in comparison with the L6 vertebral body. The long axis is in alignment with the sacrum rather than with the remaining lumbar vertebral column. The lumbosacral intervertebral disk space is narrowed (arrowhead). The sacrum appears normal (asterisk); Figure 3: A ventrodorsal lumbosacral radiograph of the dog in Figure 2. The left transverse process of the L7 vertebra is normal (long arrows). On the right side, the L7 vertebra is articulating with the wing of the ilium (small arrows). Normal sacroiliac articulation is seen on the left (asterisk).
Typical abnormalities associated with degenerative lumbosacral stenosis include spondylosis deformans, which can extend from both the ventral and lateral aspects of the L7 and sacral vertebral bodies; narrowing of the L7-S1 intervertebral disk space; sclerosis of the vertebral end plates; and ventral displacement of the sacrum in relation to the L7 vertebra (Figures 4-6).19,25,27,28
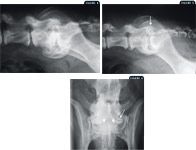
Figure 4: A lateral lumbosacral radiograph of a dog. Note the sclerosis of the vertebral end plates of the L7 vertebra and the sacrum with narrowing of the disk space as well as the ventral spondylosis. A slight ventral subluxation of the sacrum is also present; Figure 5: A lateral radiograph of the lumbosacral vertebral column. Changes are similar to those noted in Figure 4. Additionally, note the degenerative changes in the articular facets of the L7 vertebra and the sacrum, which are difficult to appreciate given the superimposition of the wings of the ilium (arrow); Figure 6: A ventrodorsal lumbosacral radiograph of the dog in Figure 4. Lateral spondylosis can be seen at the L7 sacral articulation (long arrow). Also note the narrowing of the lumbosacral intervertebral disk space (two short arrows).
Myelography and epidurography
Occasionally, contrast radiographic studies have been used to diagnose degenerative lumbosacral stenosis. Myelography requires injecting iodinated contrast agents into the subarachnoid space either at the cisterna magna or L5-L6 intervertebral space. In general, the dural sac ends between the L6 and S1 vertebrae in dogs.4 Large-breed dogs often have more cranial terminations than small-breed dogs.5 In some dogs, the dural sac extends into the sacrum, but the dural sac tends to lie more dorsally in the vertebral canal.14 As a result, ventral lesions may not be identified. Consequently, myelography generally is not helpful in diagnosing degenerative lumbosacral stenosis. Myelography does allow clinicians to evaluate the entire vertebral column for other lesions.
Epidurography requires injecting iodinated contrast media into the epidural space. Injections are made either at the L7-S1 intervertebral space, between the sacrum and first coccygeal vertebra, or between coccygeal vertebrae. It is technically easier to perform and has fewer side effects than myelography. Unfortunately, epidurograms can be difficult to interpret since it is normal to see irregular filling as the neural structures and epidural fat displace contrast. A lack of contrast on the ventral vertebral canal and compression of more than 50% of the vertebral canal are consistent with degenerative lumbosacral stenosis (Figures 7 & 8).27,29 Make sure to perform myelography before epidurography if you plan to perform both procedures. Also obtain radiographs in flexed, extended, and neutral positions to increase the likelihood of identifying a lesion.
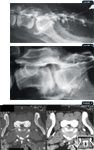
Figure 7: A lateral epidurogram of a dog. Iodinated contrast has been injected into the epidural space between the first and second coccygeal vertebra. Note the filling defect overlying the lumbosacral space consistent with intervertebral disk herniation; Figure 8: Another lateral epidurogram. Note the large filling defect overlying the lumbosacral intervertebral disk; Figure 9: Axial CT images at the level of the lumbosacral intervertebral disk space in a dog. The image on the right is a soft tissue window while the image on the left is a bone tissue window. Note the narrowing of the vertebral canal and lack of epidural fat (long white arrow), as well as ventral spondylosis deformans (short white arrows). The lack of epidural fat can be seen in the bottom right inset (black arrow).
Computed tomography
CT has largely replaced plain radiography for evaluating patients suspected of having degenerative lumbosacral stenosis. CT provides a cross-sectional evaluation of the complex anatomy and has better soft tissue resolution compared with plain radiography, thereby allowing better assessment of the diameter of the vertebral canal, articular processes, intervertebral disk, lateral recesses, and nerve roots. Abnormalities identified with CT include intervertebral disk herniation and consequent loss of epidural fat, narrowing of the intervertebral foramen, abnormalities in the articular processes, and spondylosis (Figures 9 & 10).30 Adding intravenous iodinated contrast media may enhance visualization of the anatomy of the lumbosacral articulation.31 Unfortunately, as with all other imaging modalities, the magnitude of abnormalities identified with CT does not correlate with clinical signs.32 In fact, lumbosacral stenosis has been identified in dogs without clinical signs.33
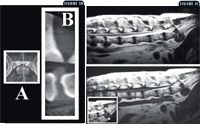
Figure 10: A sagittal plane computer reconstruction of a CT image of the lumbosacral articulation in a dog. Image A represents the plane of the reconstructed image. Image B is the sagittal reconstruction. Note the herniated intervertebral disk (cranial is to the left side of Image B); Figure 11: A sagittal plane MRI of the lumbosacral vertebral column in a dog. The top image is T2-weighted, and the bottom image is T1-weighted. A large herniation of the L7-sacral intervertebral disk is present. In the bottom left inset, the intervertebral disk herniation (arrow), ventral spondylosis (arrowhead), and sacrum (asterisk) are identified.
Magnetic resonance imaging
MRI has largely replaced other diagnostic imaging techniques as the premier imaging modality for evaluating the nervous system. As with CT, MRI provides cross-sectional images of the complex anatomy of the lumbosacral articulation. Furthermore, images can be obtained in multiple planes without losing image quality as is seen with computer-reconstructed images with CT. MRI also provides superior soft tissue discrimination compared with CT. Consequently, MRI is my preferred method of imaging the lumbosacral articulation.
MRI features in dogs with degenerative lumbosacral stenosis include degeneration of the nucleus pulposus as evidenced by a decreased signal intensity on a T2-weighted image, protrusion of the annulus fibrosis, narrowing of the vertebral canal, loss of epidural fat overlying the intervertebral disk herniation (Figure 11), compression of the L7 nerve root within the intervertebral foramen, compression of the sacral and coccygeal nerve roots, and degenerative changes in the articular processes (Figure 12).34,35 Occasionally, lateralized intervertebral disk material can be seen in the intervertebral foramen (Figure 13). Unfortunately, as with all other imaging modalities, MRI features do not necessarily correlate with clinical findings, so be careful when trying to attribute imaging results to clinical signs.32,36 Instead, imaging findings should be used to support neurologic examination findings.
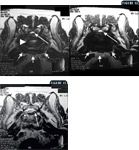
Figure 12: An axial plane MRI at the level of the lumbosacral intervertebral disk space in a dog. On the right side is a T2-weighted image and on the left is a T1-weighted image. Degeneration of the nucleus pulposus (arrowhead) is evidenced by a lack of hyperintensity on the T2-weighted image. Narrowing of the vertebral canal and loss of epidural fat are also present (long arrow). Additionally, ventral and lateral spondylosis is present (short arrows);Figure 13: An axial plane T2-weighted MRI of the level of the caudal aspect of the L7 vertebra in a dog. Note the lateralized intervertebral disk herniation within the intervertebral foramen (arrow). The normal intervertebral foramen is outlined by the arrowheads.
TREATMENT
Treatment options for degenerative lumbosacral stenosis can be divided into medical and surgical therapy.
Medical therapy
Medical therapy consists of exercise restriction and administering anti-inflammatory agents.8,12,21 Institute exercise restriction (the patient is confined and allowed out only with controlled leash walking) for a minimum of four to six weeks. Then institute a gradual return to exercise over an additional four to six weeks.
During this period, anti-inflammatory medications can be used to reduce pain. In mild cases, such as dogs with only hyperesthesia or minimum neurologic deficits, you can try nonsteroidal anti-inflammatory drugs (NSAIDs). Any of the available NSAIDs can be used at standard dosages. A specific duration of therapy is not known, but four to six weeks may be needed. In dogs with more severe neurologic deficits, you can use corticosteroids at anti-inflammatory dosages (e.g. prednisone at 0.5 to 1 mg/kg/day). The doses should be tapered over two to four weeks. Use caution when administering corticosteroids because serious side effects can include gastrointestinal ulceration, increased thirst and appetite, and muscle atrophy and weakness. Concurrent treatment with NSAIDs and corticosteroids is contraindicated.
The efficacy of medical management is variable.8,12,21 Dogs with more severe neurologic deficits or dogs that have a progressive decline in function or persistent pain in spite of medical therapy are more likely to benefit from surgical intervention.
Surgical treatment
Various surgical techniques have been used to treat degenerative lumbosacral stenosis, including dorsal laminectomy, dorsal laminectomy and diskectomy, and dorsal laminectomy and subsequent stabilization.37
Briefly, the patient is positioned in dorsal recumbency, and a dorsal midline incision is made from approximately L5 through the sacrum. The overlying epaxial musculature, which includes the multifidus lumborum and sacrocaudalis medialis dorsalis, is dissected down the midline. The L7 sacral articulation is exposed. A pneumatic drill is used to remove the dorsal lamina from the caudal aspect of the L7 vertebra through the cranial aspect of the sacrum. The interarcuate ligament is carefully removed with the dorsal lamina, exposing the epidural fat and nerve roots. The lateral borders of the laminectomy are bounded by the medial aspect of the L7-sacral articular processes. Overzealous removal of pedicles laterally can result in subluxation.
At this point, diskectomy is performed by gently retracting the exposed nerve roots laterally across the midline. A No. 65 Beaver blade or No. 11 Bard Parker scalpel is used to remove the exposed half of the dorsal longitudinal ligament and dorsal aspect of the anulus fibrosus and nucleus pulposus. Further curettage of the intervertebral disk can be performed with bone curettes. Once curettage is completed, the nerve roots are retracted to the other side, and the procedure is repeated. Before closure, subcutaneous fat is harvested and placed over the laminectomy.
Internal fixation can be combined with dorsal decompression, but this requires technical expertise with orthopedic implants. Once decompression has been achieved, internal fixation can be accomplished by placing cortical bone screws across the L7-sacrum articular processes and into the body of the sacrum.
As with any surgical procedure, postoperative care is important. Most commonly, analgesia is provided with opioid analgesics. Supportive care with intravenous fluid therapy should be provided until the patient begins eating and drinking. If the patient cannot voluntarily urinate, it is important to prevent overdistention of the urinary bladder. This can be accomplished with manual expression, intermittent sterile catheterization, or a sterile closed urinary collection system. Patients can walk slowly on a leash after surgery; however, depending on an animal's need, additional hindquarters support should be provided. Various commercially available sling supports or a towel placed under the caudal abdomen can help a patient when walking. Soft bedding and frequent repositioning help prevent the development of pressure sores.
PROGNOSIS AND OUTCOME
The long-term successful outcome of dogs undergoing surgical interventions for degenerative lumbosacral stenosis range between 69% and 94%.12,28,38-41 Likely part of the variability in the number of successful outcomes results from applying different definitions of success.38 There seem to be few preoperative prognostic indicators for return of function postoperatively. In some studies, most dogs with clinical signs limited to pain had successful outcomes.28,40 While information is available on only a small number of patients, resolution of incontinence ranges from 13% to 45% of cases.12,28,38 In one study, dogs with incontinence for less than one month had a greater likelihood of regaining continence postoperatively.38
Military working dogs present a unique population of dogs in which a greater degree of return of function is required after surgery in order for them to perform their high-activity work. Consequently, several studies have evaluated the outcome of surgical intervention in military dogs.32,41,42 Overall successful outcome ranged from 41% to 78% of military working dogs being able to return to normal function.32,41,42 Age and severity of neurologic deficits were prognostically significant factors affecting outcome postoperatively.42 Dog older than 9 years of age or dogs with severe deficits (paresis, muscle atrophy, and urinary or fecal incontinence) were unlikely to return to work activity.42
CONCLUSION
In summary, lumbosacral disease is a syndrome rather than a disease. The signs consistent with lumbosacral syndrome are the result of dysfunction of the L7, sacral, and caudal spinal cord segments or spinal nerve roots. Consequently, patients present with varying degrees of sensory and motor dysfunction to the pelvic limbs. While many conditions result in signs of lumbosacral disease, degenerative lumbosacral stenosis is relatively common. Affected patients can present with paraparesis, urinary and fecal incontinence, hyperesthesia, and abnormal tail function. Degenerative lumbosacral stenosis is the result of Hansen's Type II intervertebral disk disease, hypertrophy of the dorsal longitudinal ligament and interarcuate ligament, and degenerative changes of the articular facets at the L7-sacral articulation.
Various tests have been used to diagnose degenerative lumbosacral stenosis. MRI is now considered the premier method of imaging the lumbosacral vertebral column. Despite advanced imaging procedures, clinical signs do not always correlate with imaging findings. So care must be exercised when interpreting imaging findings. Treatment of affected dogs includes both medical and surgical procedures. The prognosis depends on several factors, including age and degree of dysfunction. Overall, many affected dogs can benefit from therapeutic interventions.
Marc Kent, DVM, DACVIM (neurology, internal medicine)
Department of Small Animal Medicine and Surgery
College of Veterinary Medicine
The University of Georgia
Athens, GA 30602
REFERENCES
1. Lenehan TM. Canine cauda equina syndrome. Compend Contin Educ Pract Vet 1983;5:941-950.
2. Tarvin G, Prata RG. Lumbosacral stenosis in dogs. J Am Vet Med Assoc 1980;177:154-159.
3. Gilmore DR. Lumbosacral diskospondylitis in 21 dogs. J Am Anim Hosp Assoc 1987;23:57-61.
4. Fletcher TM. Spinal cord and meninges. In: Evans HE, Miller ME, eds. Miller's anatomy of the dog. Philadelphia, Pa: WB Saunders, 1993:800-828.
5. Morgan JP, Atilola M, Bailey CS. Vertebral canal and spinal cord mensuration: a comparative study of its effect on lumbosacral myelography in the dachshund and German shepherd dog. J Am Vet Med Assoc 1987;191:951-957.
6. DeLahunta A. Lower motor neuron—general somatic efferent system. In: Veterinary neuroanatomy and clinical neurology. Philadelphia, Pa: WB Saunders, 1983:53-94.
7. Bailey CS, Kitchell RL, Haghighi SS, et al. Spinal nerve root origins of the cutaneous nerves of the canine pelvic limb. Am J Vet Res 1988;49:115-119.
8. Chambers JN. Degenerative lumbosacral stenosis in dogs. Vet Med Rep 1989;1:166-180.
9. Lang J, Hani H, Schawalder P. A sacral lesion resembling osteochondrosis in the German Shepherd dog. Vet Radiol Ultrasound 1992;33:69-76.
10. Hanna FY. Lumbosacral osteochondrosis: radiological features and surgical management in 34 dogs. J Small Anim Pract 2001;42:272-278.
11. Benninger MI, Seiler GS, Robinson LE, et al. Three-dimensional motion pattern of the caudal lumbar and lumbosacral portions of the vertebral column of dogs. Am J Vet Res 2004;65:544-551.
12. Oliver JE Jr, Selcer RR, Simpson S. Cauda equina compression from lumbosacral malarticulation and malformation in the dog. J Am Vet Med Assoc 1978;173:207-214.
13. Mattoon JS, Koblik PD. Quantitative survey radiographic evaluation of the lumbosacral spine of normal dogs and dogs with degenerative lumbosacral stenosis. Vet Radiol Ultrasound 1993;34:194-206.
14. Lang J. Flexion-extension myelography of the canine cauda equina. Vet Radiol Ultrasound 1988;29:242-257.
15. Slocum B, Devine T. L7-S1 fixation-fusion for treatment of cauda equina compression in the dog. J Am Vet Med Assoc 1986;188:31-35.
16. Bagley RS. Surgical stabilization of the lumbosacral joint. In: Slatter DH, ed. Textbook of small animal surgery. Philadelphia, Pa: WB Saunders, 2003:1238-1243.
17. Jones JC, Hudson JA, Sorjonen DC, et al. Effects of experimental nerve root compression on arterial blood flow velocity in the seventh lumbar spinal ganglion of the dog: measurement using intraoperative Doppler ultrasonography. Vet Radiol Ultrasound 1996;37:133-140.
18. Wheeler SJ. Lumbosacral disease. Vet Clin North Am Small Anim Pract 1992;22:937-950.
19. Indrieri RJ. Lumbosacral stenosis and injury to the cauda equina. Vet Clin North Am Small Anim Pract 1988;18:697-710.
20. Schulman AJ. Canine cauda equina syndrome. Compend Contin Educ Pract Vet 1988;10:835-844.
21. Ness MG. Degenerative lumbosacral stenosis in the dog: a review of 30 cases. J Small Anim Pract 1994;35:185-190.
22. Glass EN, Kent M. The clinical examination for neuromuscular disease. Vet Clin North Am Small Anim Pract 2002;32:1-29.
23. Palmer RH, Chambers JN. Canine lumbosacral disease. Part I. Anatomy, pathophysiology, and clinical presentation. Compend Contin Educ Pract Vet 1991;13:61-68.
24. Sisson AF, LeCouteur RA, Ingram JT, et al. Diagnosis of cauda equina abnormalities by using electromyography, discography, and epidurography in dogs. J Vet Intern Med 1992;6:253-263.
25. Morgan JP, Bailey CS. Cauda equina syndrome in the dog. J Small Anim Pract 1990;31:69-76.
26. Morgan JP, Bahr A, Franti CE, et al. Lumbosacral transitional vertebrae as a predisposing cause of cauda equina syndrome in German shepherd dogs: 161 cases (1987-1990). J Am Vet Med Assoc 1993;202:1877-1882.
27. Selcer BA, Chambers JN, Schwensen K, et al. Epidurography as a diagnostic aid in canine lumbosacral compressive disease: 47 cases (1981-1986). Vet Comp Orthop Traumatol 1988;2:97-103.
28. Chambers JN, Selcer BA, Oliver JE Jr. Results of treatment of degenerative lumbosacral stenosis in dogs by exploration and excision. Vet Comp Orthop Traumatol 1988;1:130-133.
29. Roberts RE, Selcer BA. Myelography and epidurography. Vet Clin North Am Small Anim Pract 1993;23:307-329.
30. Jones JC, Sorjonen DC, Simpson ST, et al. Comparison between computed tomographic and surgical findings in nine large-breed dogs with lumbosacral stenosis. Vet Radiol Ultrasound 1996;37:247-256.
31. Jones JC, Shires PK, Inzana KD, et al. Evaluation of canine lumbosacral stenosis using intravenous contrast-enhanced computed tomography. Vet Radiol Ultrasound 1999;40:100-114.
32. Jones JC, Banfield CM, Ward DL. Association between postoperative outcome and results of magnetic resonance imaging and computed tomography in working dogs with degenerative lumbosacral stenosis. J Am Vet Med Assoc 2000;216:1769-1774.
33. Jones JC, Inzana KD. Subclinical CT abnormalities in the lumbosacral spine of older large-breed dogs. Vet Radiol Ultrasound 2000;41:19-26.
34. deHann JJ, Shelton SB, Ackerman N. Magnetic resonance imaging in the diagnosis of degenerative lumbosacral stenosis in four dogs. Vet Radiol Ultrasound 1993;22:1-4.
35. Adams WH, Daniel GB, Pardo AD, et al. Magnetic resonance imaging of the caudal lumbar and lumbosacral spine in 13 dogs (1990-1993). Vet Radiol Ultrasound 1995;36:3-13.
36. Mayhew PD, Kapatkin AS, Wortman JA, et al. Association of cauda equina compression on magnetic resonance images and clinical signs in dogs with degenerative lumbosacral stenosis. J Am Anim Hosp Assoc 2002;38:555-562.
37. Sharp NJH, Wheeler SJ. Lumbosacral disease. In: Small animal spinal disorders diagnosis and surgery. Edinburgh: Elsevier Mosby, 2005:181-210.
38. De Risio L, Sharp NJ, Olby NJ, et al. Predictors of outcome after dorsal decompressive laminectomy for degenerative lumbosacral stenosis in dogs: 69 cases (1987-1997). J Am Vet Med Assoc 2001;219:624-628.
39. Danielsson F, Sjostrom L. Surgical treatment of degenerative lumbosacral stenosis in dogs. Vet Surg 1999;28:91-98.
40. Watt P. Degenerative lumbosacral stenosis in 18 dogs. J Small Anim Pract 1991;32:125-134.
41. Janssens F, Moens Y, Coppens P. Lumbosacral degenerative stenosis in the dog, the results of dorsal decompression with dorsal annulectomy and nuclectomy. Vet Comp Orthop Traumatol 2000;13:97-103.
42. Linn LL, Bartels KE, Rochat MC, et al. Lumbosacral stenosis in 29 military working dogs: epidemiologic findings and outcome after surgical intervention (1990-1999). Vet Surg 2003;32:21-29.