Endoscopy Brief: Identifying the cause of acute cough and respiratory distress in a toy poodle
A 13-year-old 12-lb (5.5-kg) intact male toy poodle was presented to Mississippi State University's College of Veterinary Medicine for evaluation of coughing and increased respiratory effort and exercise intolerance of four days' duration.
A 13-year-old 12-lb (5.5-kg) intact male toy poodle was presented to Mississippi State University's College of Veterinary Medicine for evaluation of coughing and increased respiratory effort and exercise intolerance of four days' duration. The dog's vaccinations, heartworm preventive administration, and negative heartworm test results were current.
At initial presentation, the dog was bright and alert. Its hydration status and temperature were normal; its heart rate (160 beats/min) and respiratory rate (54 breaths/min) were elevated. A grade I/VI systolic heart murmur was auscultated, with the point of maximum intensity over the mitral valve area. Pulmonary auscultation revealed bilateral crackles cranioventrally. A cough could be frequently elicited with tracheal palpation. The patient's respiratory distress worsened during the physical examination, and the patient was placed in an oxygen cage at 40% oxygen after initiation of intravenous fluids administered at a maintenance rate. Pulse oximetry revealed an SpO2 of 90%.
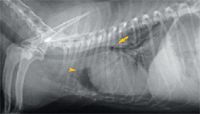
Figure 1. A right lateral thoracic radiograph at presentation. Mild generalized cardiomegaly is causing dorsal displacement of the tracheal bifurcation (arrow). Increased soft tissue opacity can be seen cranial to the cardiac silhouette (arrowhead).
A complete blood count revealed leukocytosis (total WBC count = 21,180/μl; reference range = 6,000 to 17,000/μl) with neutrophilia (15,190/μl segmented neutrophils; reference range = 3,000 to 11,500/μl) and a left shift (1,302/μl band neutrophils; reference range = 0 to 300/μl). A serum chemistry profile revealed no significant abnormalities. Thoracic radiographs revealed mild generalized cardiomegaly (Figures 1 & 2). Radiographic evidence of heart failure was not seen, and the heart enlargement was not considered severe enough to compress the mainstem bronchi and contribute to the cough. Inspiratory and expiratory lateral views revealed no evidence of tracheal collapse. On the right lateral thoracic radiograph, an area of increased soft tissue opacity was seen cranial to the heart. On the ventrodorsal view, this radiopacity was in the region of the left cranial lung lobe. Closer evaluation revealed both interstitial and alveolar lung patterns in this region. A single air bronchogram appeared to be traversing the long axis of the left cranial lung lobe. Radiographic findings were compatible with consolidation of the left cranial lung lobe.
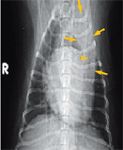
Figure 2. A ventrodorsal thoracic radiograph at presentation. There is an intense, focal alveolar pattern in the left cranial lung lobe (arrows), which contains a prominent air bronchogram (arrowhead). Mild generalized cardiomegaly is present.
The differential diagnoses for these findings include lobar pneumonia, pulmonary neoplasia, and, much less likely, lung lobe torsion. Bronchoscopy and a bronchoalveolar lavage were performed to further assess the lung abnormalities.
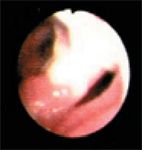
Figure 3. The tracheal bifurcation during expiration. The partially collapsed left mainstem bronchus is on the right side of the image.
BRONCHOSCOPIC EXAMINATION AND INITIAL THERAPY
After partial patient stabilization (respiratory rate = 20 breaths/min and spo2 = 97% with concurrent oxygen therapy), the patient was anesthetized and positioned in sternal recumbency. A 5-mm-diameter video bronchoscope (Olympus BF Type P20D—Olympus) was used to examine the oral cavity, pharynx, and trachea. No abnormalities were found. The bronchoscope was then advanced to the tracheal bifurcation.
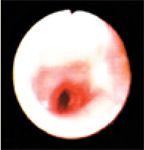
Figure 4. The left cranial lobar and caudal segmental bronchi of the left cranial lung lobe during inspiration. The bronchi are moderately erythematous.
The left mainstem bronchus appeared erythematous and inflamed, and the lumen was narrowed (Figure 3) during both phases of respiration but more so during expiration. The left cranial lobar and caudal segmental bronchi of the left cranial lung lobe were visualized, as shown during inspiration (Figure 4) and expiration (Figure 5). The left cranial lobar and caudal segmental bronchi were erythematous and completely collapsed with expiration. The right mainstem bronchus was examined next and appeared normal. However, evaluation of the right middle and accessory bronchi revealed partial collapse during expiration (no gross evidence of inflammation was noted in these bronchi). Figures 6 and 7 show both the right caudal and accessory bronchi during inspiration and expiration, respectively.

Figure 5. The left cranial lobar and caudal segmental bronchi of the left cranial lung lobe during expiration. There is complete collapse and moderate erythema of both bronchi.
Bronchoalveolar lavage was performed, and lavage samples from the bronchus of the left cranial lung lobe and the right mainstem bronchus were collected and submitted for cytologic examination, bacterial culture, and antimicrobial sensitivity testing.
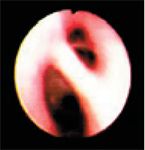
Figure 6. The right caudal (left side of image) and accessory bronchi during inspiration. No abnormalities are identified.
The dog recovered from anesthesia without complications, and treatment was started with intravenous fluids administered at a maintenance rate and intravenous broad-spectrum antibiotics. The patient was placed in an oxygen cage at 40% oxygen and was nebulized with sterile 0.9% saline solution for 15 minutes every eight hours, followed by gentle coupage.
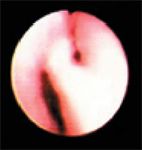
Figure 7. The right caudal (left side of image) and accessory bronchi during expiration. There is partial collapse of both bronchi.
DIAGNOSIS AND FOLLOW-UP CARE
Cytologic analysis of the bronchoalveolar lavage samples revealed normal findings (primarily alveolar macrophages) from the right side. Severe purulent inflammation (many neutrophils and macrophages but no neoplastic cells or microorganisms) was identified in the lavage sample from the left cranial lung lobe. These results, coupled with the findings from the bronchoscopy, led us to a presumptive diagnosis of bronchopneumonia and lower airway collapse.
Bacterial cultures yielded Enterobacter cloacae growth from the left and right mainstem bronchial lavages. The patient was weaned off of oxygen and nebulization therapy on the second and third days after admission and discharged on the fourth day with broad-spectrum antibiotics based on the culture and sensitivity results. The owners were instructed to return for a recheck every two weeks until the pneumonia resolved.
Recheck examinations at two and four weeks revealed that the dog had continued clinical and radiographic improvement. Thoracic radiographs at the 10-week recheck showed resolution of the pneumonia (Figures 8 & 9). Infrequent coughing was still present and was thought to be caused by continued bronchial collapse and subsequent airway irritation, so an albuterol inhaler was prescribed to help decrease expiratory effort and potentially reduce bronchial collapse. Antibiotic therapy was discontinued. Four weeks after initiating the albuterol inhaler, the owner reported that the dog had decreased frequency of coughing.
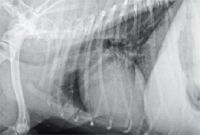
Figure 8. A right lateral thoracic radiograph taken three months after initial presentation showing resolution of the previously identified increased soft tissue opacity cranial to the heart. The previously described generalized cardiomegaly is relatively unchanged.
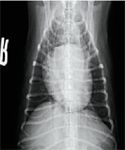
Figure 9. A ventrodorsal thoracic radiograph taken three months after initial presentation showing resolution of the previously identified alveolar pattern and unchanged generalized cardiomegaly.
The final diagnosis was bacterial pneumonia and lung lobe consolidation caused by E. cloacae. The relationship between the lobar pneumonia and the bronchial collapse is unclear. The pneumonia may have been secondary to primary bronchial collapse and a resulting decrease in mucocilliary clearance; however, primary lower airway disease can cause secondary lower airway collapse due to alterations in intrathoracic pressures. Chronic bronchitis can predispose a patient to both pneumonia and lower airway collapse, but this dog's history and radiographic evidence did not support this scenario. Because the patient was lost to follow-up, the cause of the bronchial collapse could not be pursued.
DISCUSSION
Differential diagnoses in dogs with coughing and respiratory difficulty are many. Some of the more common differential diagnoses in older, small-breed dogs include cardiogenic causes (e.g. mitral insufficiency with left atrial enlargement and pressure on the left mainstem bronchus, cardiogenic pulmonary edema, heartworms) and airway or parenchymal causes (e.g. collapsing trachea, infectious tracheobronchitis, chronic bronchitis, neoplasia, pneumonia, pulmonary thrombosis).
Dogs with bronchopneumonia often present with signs of lower respiratory disease such as coughing (more common in dogs than cats), exercise intolerance, respiratory distress, and nasal discharge. However, the only identifiable clinical signs may be subtle—depression, weight loss, and decreased appetite may be all that are present. Increased lung sounds may be heard over the affected lung fields (dependent lung fields are usually more severely affected). Fever may or may not be present.1
A complete blood count may show a stress leukogram or a neutrophilic leukocytosis with or without a left shift. The classic inflammatory leukogram may not be present.1 Thoracic radiographs may show an interstitial pattern early in the disease process or an alveolar pattern as the disease progresses. Diagnostic confirmation of bacterial pneumonia is based on results of bacterial culture and antimicrobial sensitivity testing of lower airway and alveolar secretions obtained by tracheal wash or bronchoalveolar lavage. Common pathogens isolated from the lower respiratory tract include Escherichia coli, Pasteurella species, Klebsiella pneumoniae, Bordetella bronchiseptica, Pseudomonas aeruginosa, and Staphylococcus and Streptococcus species.2 Species belonging to the Enterobacteriaceae family have also been commonly reported.3 Anaerobic cultures should also be performed, and Mycoplasma species cultures should be considered, especially in young dogs and cats.1
In cases of bacterial pneumonia, a predisposing abnormality often exists. Examples of such abnormalities include decreased clearance of inhaled debris from the airways, such as that which occurs in patients with chronic bronchitis, tracheal or bronchial collapse, bronchiectasis, or ciliary dyskinesia. Immunosuppression (caused by drugs, malnutrition, stress, endocrinopathies, viral infections), aspiration, inhaled foreign bodies, neoplasia, fungal disease, and pulmonary parasites can also predispose an animal to bacterial pneumonia.2 Indwelling intravenous, arterial, and urinary catheters can increase the risk of bacterial pneumonia.1 Most cases of bacterial pneumonia result from bacterial entry through the airways.1 This route of infection most commonly affects the dependent airways, assuming a cranioventral pattern.1 Bacterial pneumonia of a hematogenous origin often presents as a caudal or diffuse pattern with increased interstitial involvement.1
The treatment of bacterial pneumonia consists primarily of supportive care and appropriate antibiotic therapy. Significantly more patients respond to antibiotics based on results of bacterial culture and antimicrobial sensitivity testing than on empirical therapy.4 Reasonable initial antibiotic choices pending the results of sensitivity testing include cephalexin, trimethoprim-sulfadiazine, chloramphenicol, or amoxicillin trihydrate-clavulanate potassium. If the infection is life-threatening, as with concurrent sepsis, intravenous treatment with broad-spectrum antibiotics is indicated. Aerosol administration of antibiotics by nebulization can be used in addition to systemic therapy.1
Use intravenous fluids to maintain adequate hydration of the airways and to facilitate mucociliary clearance. It is important to note that diuretics counteract airway hydration, so they should not be used. Airway humidification with a nebulizer or vaporizer for 15 to 20 minutes, three to four times a day, also aids mucociliary clearance, as does frequent coupage. Supplemental oxygen may be needed in patients with low oxygen partial pressure.5 The use of bronchodilators is controversial, and corticosteroids and cough suppressants should be avoided.1
Specific indications for bronchoscopy include chronic coughing, hemoptysis, unexplained pulmonary infiltrates, and suspicion of an airway mass or foreign body. Bronchoscopy allows visualization of dynamic airway changes such as tracheal or bronchial collapse6 and foreign body retrieval. Also, lower airway samples for cytologic examination and culture can be obtained by brush cytology or by performing a bronchoalveolar lavage. Samples collected by bronchoscopy for cytologic examination and culture consistently have a greater diagnostic sensitivity than those obtained with a transtracheal wash.7,8
Since bronchoscopy requires general anesthesia, patients with respiratory distress often cannot safely undergo the procedure. Patients with advanced cardiopulmonary disease or marked metabolic derangements are also at higher risk during any procedure that involves general anesthesia.
Bronchoscopy is best performed by using a flexible fiberoptic endoscope or videoendoscope. Most bronchoscopy procedures in dogs and cats can be performed with a 5-mm-diameter, 55-mm-long endoscope; however, small patients may require a 3.7-mm-diameter endoscope. A three-dimensional map of the endobronchial anatomy of the veterinary patient is especially helpful in navigating the bronchoscope.9,10 Bronchoscopy can be performed with inhalation anesthesia if the endotracheal tube lumen is large enough to allow passage of the bronchoscope without occluding gas flow through the endotracheal tube. For smaller endotracheal tubes, injectable anesthesia or repeated temporary intubation must be used. The biopsy channel of the bronchoscope can be used to deliver oxygen during the procedure, pass biopsy forceps or cytology brushes, and perform bronchoalveolar lavage.6 Culture samples can be obtained from the bronchoalveolar lavage fluid or by using a guarded culturette, which can be passed through the biopsy channel (Figure 10).9

Figure 10. A guarded culturette (Boston Scientific Microvasive microbiology specimen brush) with a carbon wax plug (arrow) that expels just before sampling to help reduce the risk of contamination.
"Endoscopy Brief" was contributed by William R. Lee, DVM; John W. Tyler, DVM, DACVIM; and H. Dan Cantwell, DVM, MS, DACVR, Department of Clinical Sciences, College of Veterinary Medicine, Mississippi State University, Mississippi State, MS 39762. Dr. Lee's current address is College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27695-8401.
REFERENCES
1. Hawkins EC. Pulmonary parenchymal diseases. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine. 5th ed. Philadelphia, Pa: WB Saunders Co, 2000;1061-1089.
2. Hawkins EC. Disorders of the pulmonary parenchyma. In: Nelson RW, Couto CG, eds. Small animal internal medicine. 2nd ed. St. Louis, Mo: Mosby-Year Book, 1998;125.
3. Angus JC, Jang SS, Hirsh DC. Microbiological study of transtracheal aspirates from dogs with suspected lower respiratory tract disease: 264 cases. J Am Vet Med Assoc 1997;210:55-58.
4. Thayer GW, Robinson S. Bacterial bronchopneumonia in the dog: A review of 42 cases. J Am Anim Hosp Assoc 1984;20:731.
5. Ford RB. Bacterial pneumonia. In: Bonagura JD, ed. Kirk's current veterinary therapy XIII small animal practice. Philadelphia, Pa: WB Saunders Co, 2000;812-815.
6. Johnson LJ. Small animal bronchoscopy. Vet Clin North Am Small Anim Pract 2001;31:691-705.
7. Hawkins EC, DeNicola DB. Cytologic analysis of tracheal wash specimens and bronchoalveolar lavage fluid in the diagnosis of mycotic infection in dogs. J Am Vet Med Assoc 1990;197:79-83.
8. Hawkins EC, Morrison WB, DeNicola DB, et al. Cytologic analysis of bronchoalveolar lavage fluid from 47 dogs with multicentric malignant lymphoma. J Am Vet Med Assoc 1993;203:1418-1425.
9. McKiernan BC. Bronchoscopy. In: McCarthy TC, ed. Veterinary endoscopy for the small animal practitioner. Philadelphia, Pa: WB Saunders Co, 2005;201-227.
10. Amis TC, McKiernan BC. Systematic identification of endobronchial anatomy during bronchoscopy in the dog. Am J Vet Res 1986;47:2649-2657.