The expanding universe of three parasites
Are these three emerging parasites on your radar?
You have undoubtedly heard it before, but transporting our pets around the world is one of the most significant factors in the emergence of diseases in new geographic areas. This article discusses three primarily canine parasites: Angiostrongylus vasorum, Heterobilharzia americana, and Trypanosoma cruzi. Two of these parasites are already spreading within the United States, while the third is not yet present but is knocking at the door.
ANGIOSTRONGYLUS VASORUM
Canine pulmonary angiostrongylosis is caused by the nematode A. vasorum.1-3 The first anecdotal reports of canine pulmonary angiostrongylosis were in France in 1813 and 1833. Subsequent research on this nematode in France, coupled with endemic foci identified in southwestern France, led to this parasite's common name—the French heartworm. No longer limited to France, the distribution of this parasite is worldwide. Recent reports of canine pulmonary angiostrongylosis in Newfoundland and Canada,4,5 as well as in Scotland6 and elsewhere indicate this parasite continues to spread (see the sidebar titled "Angiostrongylus vasorum: Spreading in North America").

Angiostrongylus vasorum: Spreading in North America
Life cycle
Compared with Dirofilaria immitis, which are 120 to 310 mm, A. vasorum adults are small (14 to 20.5 mm).1 Like that of D. immitis, the life cycle of A. vasorum is indirect, but snails and slugs are the intermediate hosts rather than mosquitoes.1-3 Both male and female A. vasorum live in the right side of the canine heart and pulmonary arteries (Figure 1), although with aberrant migration, they can end up in other areas (eyes, kidneys, brain, pancreas, femoral artery).1-3
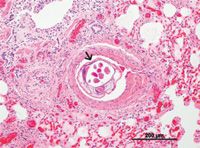
1. A histologic section of canine lung with adult Angiostrongylus vasorum present (arrow) (hematoxyllin and eosin stain; 10X). (Photo courtesy of Dr. Gary Conboy, University of Prince Edward Island.)
The females lay eggs that lodge in smaller capillaries where they develop and hatch (Figure 2). The hatched first-stage larvae (L1) penetrate capillaries and alveoli and migrate into larger airways where they are eventually coughed up, swallowed, and passed in the feces. They then infect a suitable gastropod intermediate host where development to the infective third-stage larvae (L3) occurs in as little as 16 to 18 days.
More than 25 species of slugs and terrestrial and aquatic snails have been shown to be suitable intermediate hosts. Dogs become infected by ingesting the infected gastropod intermediate hosts. Spontaneous expulsion of viable L3 from gastropods also occurs, indicating dogs may become infected by direct ingestion of the L3. Experimentally, Nile rats can develop patent infections, and frogs have been shown to be both intermediate and paratenic hosts. However, their relative importance to the maintenance of the life cycle is unclear.
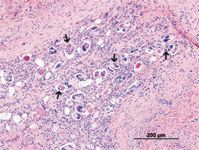
2. A histologic section of canine lung showing numerous eggs (several indicated by arrows) and L1 of Angiostrongylus vasorum (hematoxyllin and eosin stain; 10X). (Photo courtesy of Dr. Gary Conboy, University of Prince Edward Island.)
Once ingested by the dog, the L3 penetrate the gastrointestinal tract wall, migrate to visceral lymph nodes, and develop into immature adult nematodes. They then migrate to the right ventricle and pulmonary arteries via the portal circulation. The prepatent period is around 38 to 57 days, although longer prepatent periods may also occur. The adult nematodes are long-lived (at least five years), and the dog may remain infected for life.
Reservoir hosts for A. vasorum include other canids, such as red foxes, wolves, coyotes, and jackals. This parasite has also been reported in lynx, Eurasian badgers, and red pandas.1-3,7
Signalment
In Europe, there does not appear to be any sex or breed disposition, although one study did show Cavalier King Charles spaniels and Staffordshire bull terriers were overrepresented among dogs with canine pulmonary angiostrongylosis compared with the control group.3,8 In contrast, most dogs with canine pulmonary angiostrongylosis in Newfoundland are beagles used for rabbit hunting.1
Canine pulmonary angiostrongylosis has been reported in dogs as young as 3 months old and as old as 14 years; however, more than half the European cases have been reported in dogs ≤ 1 year, whereas the median age in Newfoundland cases is 4.25 years.1,2
Clinical findings
Clinically, infected dogs may be asymptomatic or minimally affected or have severe disease. Classic canine pulmonary angiostrongylosis usually presents with respiratory signs, including dyspnea and coughing.1,2,4,6,8 Crackles may be present in severe cases. Dogs with chronic infections can have pulmonary hypertension, cor pulmonale, and subsequent systolic heart murmur. Other signs may include depression, exercise intolerance, anorexia, weight loss, vomiting, or diarrhea. Severe coagulation disorders have also been identified in chronically infected dogs.1,2,9 Petechial and ecchymotic hemorrhages, hematomas (both traumatic and surgically induced), epistaxis, hemoptysis, intracranial hemorrhages, hematuria, and gastrointestinal bleeding have all been reported.1,2,9 Thrombocytopenia, prolonged activated partial thromboplastin and prothrombin times, the presence of fibrin degradation products, hyperglobulinemia, anemia, or Factor V deficiency are associated with these cases.1,2,9 Thus, a consumptive coagulopathy is indicated, but the mechanisms involved are not defined.
Diagnosis
Because the clinical spectrum of canine pulmonary angiostrongylosis is not unique, many differential diagnoses are usually considered, including viral respiratory disease, immune-mediated thrombocytopenia, and other cardiopulmonary parasites. Thus, an initial work-up, based on the severity of signs, has been described.2
Parasitologic diagnosis depends on finding L1 (Figure 3) in the feces by using the Baermann techinque.1,2 Because of the sporadic shedding of larvae in the feces, it is recommended that samples be collected on three consecutive days to enhance detection. The L1 can also be found in direct fecal smears, particularly if clinical signs are moderate to severe or recovered by tracheal wash or bronchoalveolar lavage. Angiostrongylus vasorum L1 are 310 to 399 µm in length with an anterior cephalic button and an S-shaped tail (severe kink) with a dorsal spine. These features distinguish A. vasorum L1 from those of other canine parasites, such as Crenosoma vulpis or Strongyloides stercoralis.
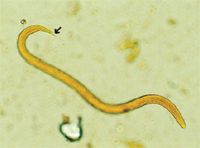
3. First-stage larva of Angiostrongylus vasorum, stained with Lugolâs iodine. Note the kinked-tail at the posterior end with the short, dorsal spine (arrow). This characteristic will differentiate first-stage larvae of A. vasorum from those of Crenosoma vulpis and Strongyloides stercoralis (40X). (Photo courtesy of Dr. Gary Conboy, University of Prince Edward Island.)
Treatment
Several anthelmintics and therapeutic regimens have been used to treat canine pulmonary angiostrongylosis. Recommendations include milbemycin oxime (0.5 mg/kg once a week for four weeks), topical moxidectin (2.5 mg/kg; imidacloprid-moxidectin spot-on combination product applied once), or fenbendazole (20 to 50 mg/kg daily for five to 21 days).1
Not every treatment is 100% efficacious; thus, follow-up fecal examinations should be conducted three to seven days after completion of the treatment regimen. An additional three-day Baermann test should be conducted again at three months and then twice a year. If no larvae are found, then further testing is performed if suspicious clinical signs recur.2 Resolution of clinical disease but not infection can occur; in these cases, retreatment is necessary.
Post-treatment complications may occur, such as severe dyspnea or ascites. Strict cage rest for the initial two or three days of treatment is recommended. The use of additional supportive therapy, such as antibiotics, immunosuppressive doses of corticosteroids, and bronchodilators, will depend on the clinical presentation.2
Prevention
In endemic and hyperendemic areas, monthly anthelmintic prophylaxis may be considered. Although adult nematodes were not found after topical moxidectin was administered at four days or 32 days postinfection,1 protocols for its, or any other anthelmintic's use, have not been established or verified. Removing feces will be a tremendous help, as it will break the life cycle and reduce environmental contamination. Owner education regarding the means by which dogs become infected and how off-leash walking of dogs contributes to their risk is advised.2 Finally, monitoring by using Baermann tests should be incorporated into the yearly wellness examination to help identify infected but asymptomatic animals.
HETEROBILHARZIA AMERICANA
Canine schistosomiasis is not an exotic disease. Rather, it is caused by the digenetic trematode H. americana. Its geographic distribution in the United States originally included the southern Atlantic Coast and the Gulf Coast but has now been documented as far north as Kansas. Thus, the distribution of the parasite appears to be the central and southeastern United States.10,11
Life cycle
Unlike most trematodes, H. americana has separate sexes. The mature trematodes live in the mesenteric veins, where they mate and the female produces eggs. Eggs in the terminal mesenteric veins penetrate through the vessel, entering the intestinal wall. After traversing the wall, they are released into the lumen and are passed with feces. The miracidium is fully developed by the time the egg enters the external environment. If the egg contacts water, the miracidium hatches and enters a freshwater snail. Asexual reproduction occurs, producing cercariae. These emerge from the snail beginning as early as 25 days after infection. Cercariae penetrate the intact skin of the definitive host and migrate through the lungs to the liver. The trematodes grow and mature in about 40 days and then migrate to the mesenteric veins. The prepatent period is about 68 days.
There are numerous reservoir hosts for H. americana, but raccoons and nutria appear to be the primary ones. Dogs are the most important domestic definitive host, but red wolves and coyotes may be important wild canid reservoirs. Experimental or natural infections with H. americana have been demonstrated in two species of aquatic snails; however, little information is known about whether additional species can be competent intermediate hosts.
Clinical findings
Passage of the eggs through the tissues provokes a severe granulomatous reaction that is responsible for most of the clinical signs.11,12 A wide spectrum of disease and presentations occurs in dogs, ranging from subclinical to granulomatous intestinal disease, granulomatous liver disease, or renal failure induced by the hypercalcemia that can result from granulomatous disease. The most common clinical findings include lethargy, weight loss, anorexia, hyporexia, vomiting, hypercalcemia, diarrhea, and polyuria and polydipsia.12 In severe infections, hepatic disease may also be present.
Complete blood count and serum chemistry profile results are often normal, although hypercalcemia with decreased serum parathyroid hormone or increased serum parathyroid hormone-related protein concentrations may be present. The hypercalcemia in these cases may be misdiagnosed as neoplasia and hypercalcemia of malignancy. Thus, canine schistosomiasis should be included on the differential diagnosis list, particularly in endemic areas, for dogs presenting with unexplained glomerulonephritis or weight loss, gastrointestinal or liver disease, or hypercalcemia.13-15
Diagnosis
Clinical diagnosis generally comes from the demonstration of the eggs in feces or in intestinal or hepatic biopsy samples. Routine fecal flotations will not detect eggs since they do not readily float. Also, if placed in water, the eggs will hatch within minutes. Thus, fecal sedimentation with 0.85% saline solution is the diagnostic technique of choice for demonstrating these eggs. The examination of direct saline smears may also reveal the presence of eggs.
As with other parasites, eggs are passed intermittently in feces, so multiple fecal examinations may be required. The identity of suspect eggs can be confirmed by resuspending eggs in deionized water, which allows them to hatch, or through a polymerase chain reaction assay. An antigen-capture ELISA is an additional diagnostic choice.11
Treatment
Both praziquantel (25 mg/kg two or three times a day for two or three days) and fenbendazole (40 to 50 mg/kg once a day for 10 days) have been used to treat this infection with variable results.11 In at least one study, hypercalcemia did not resolve unless the animals had been treated with praziquantel.12 The prognosis is good even in the presence of hypercalcemic-induced renal failure or ascites.12
TRYPANOSOMA CRUZI
Trypanosoma cruzi is a protozoan parasite that causes Chagas disease, or American trypanosomiasis. The parasite is endemic throughout Mexico and Central and South America, and an estimated 7.7 million people are infected, with 3 to 3.3 million symptomatic cases and an additional 108.6 million people at risk.16 However, with the increased movement of people from endemic areas, tourism, and pet travel, Chagas disease has become an important public health issue in the United States. Even though human prevalence within the United States is low, the blood supply is now routinely tested for evidence of Chagas infection because the parasite can be transmitted through blood transfusion and organ transplantation.17,18
Life cycle
Chagas disease is primarily a vector-borne disease, although transmission routes, in addition to those mentioned previously, also include vertical transmission (mother to fetus) and ingestion.16
Indirect transmission involves mammalian definitive hosts and triatomine bug intermediate hosts, such as assassin bugs. Mammalian hosts within the United States include opossums, wood rats, raccoons, armadillos, and coyotes.17,19 An infected triatomine bug will defecate as it feeds and pass organisms in the feces. Trypanosoma cruzi can then invade various cells at the bite wound site, form into amastigotes, and undergo asexual reproduction. They then transform in a nonreproducing stage in which they leave the cell and either invade new tissues or are ingested by a different triatomine bug as it takes a blood meal. If not ingested by an intermediate host, they transform again into intracellular amastigotes, reproducing in reticuloendothelial, neural, and glial cells and cardiac and smooth muscle cells.
Within the newly infected intermediate host, the parasite multiplies and undergoes metamorphosis into a new form in the hindgut. When the bug feeds again on a different animal, it will defecate as it feeds, and the parasite is transmitted in the feces. It then enters the body by penetrating the oral, nasal, or conjunctival mucosa or by rubbing the infectious bug feces into abrasions, such as when the bug bite site is scratched. Ingestion of an infected bug will also result in transmission of the organism.20
Signalment
The age range of clinical cases in dogs has been reported to be 6 weeks to 13 years, with about half the cases in animals < 1 year old. No sex predilection has been reported. Cases have been reported in 48 breeds of dogs, with most in the sporting group, likely as a result of lifestyle factors. Cases have also been reported in dogs from both urban and rural areas.19
Clinical findings
Acute disease in dogs is characterized by lymphadenopathy and clinical signs associated with acute myocarditis, such as pale mucous membranes, lethargy, ascites, and tachyarrhythmia.19,20 Dogs surviving the acute phase enter the indeterminate phase characterized by the lack of clinical signs. Circulating parasites can only be demonstrated by blood culture or xenodiagnosis, which is when uninfected intermediate hosts are purposefully fed on presumed infected animals and subsequently examined for the presence of the organism. An electrocardiogram is usually normal at this stage, although ventricular-based arrhythmias can be induced with exercise.20 Some dogs may then progress to chronic disease with clinical signs related to congestive myocardial failure.19,20
In a recent report characterizing Chagas disease in dogs, about half of dogs < 1 year of age presented with acute death.19 In nonacute death cases, the duration of apparent illness ranged from one day to six weeks and depended on when the client sought veterinary attention. Cardiac dysfunction, represented by cardiac enlargement and myocarditis, was the primary problem reported in both puppies and adults. Conduction disturbances, including premature ventricular contractions and atrial fibrillation, were reported in about one-fifth of the animals.
Diagnosis and treatment
Diagnosing Chagas disease is difficult. The first step is to suspect the infection. Chagas disease should be included on the differential diagnosis list for dogs presenting with signs of myocarditis or cardiomyopathy, particularly in endemic areas or if a dog has lived at any time—even years before presentation—in an endemic area.20 Diagnosis has traditionally depended on demonstration of parasites in peripheral blood or amastigotes in tissue biopsy samples.
Several serologic and molecular methods are also available, which may be particularly useful during the indeterminate and chronic phases.20-23 Immunochromatographic assays for the detection of antibodies in dogs in-clinic have been described and appear useful as screening tools.21-23 The primary problem with most serologic methods is the cross-reaction with Leishmania infantum, another protozoan parasite endemic to the same areas of the United States.20,23 Recommendations are to confirm screening tests with another diagnostic method available through specialized laboratories.
Because most cases are diagnosed during the chronic stage, treatment is unrewarding. Supportive cardiac therapy becomes the mainstay of treatment.20
Prevention
Recommendations aimed at preventing infections include limiting contact with vectors and wild reservoir hosts. Although the parasite is usually transmitted in the feces of the vector, it is thought that most infections in dogs in the United States are acquired through the ingestion, which releases the organisms into the mouth of the dog. Use of integrated pest management methods, such as changing outside lighting, housing dogs indoors at night, and optimizing pesticide regimens, should be used to reduce contact with vectors.19,20 Additionally, dogs should not be fed fresh meat from reservoir hosts.19,20 Removing T. cruzi antibody-positive females from the breeding stock may reduce vertical transmission. Dogs used as blood donors should also be screened to determine their status.
Dogs are more competent definitive hosts than either cats or people are and are considered important reservoir hosts in Central and South America.19 However, the risk of acquiring infection from an infected dog is thought to be extremely low in the United States at this time.20 Nevertheless, because infections can be passed through blood, veterinarians and their staffs need to be especially careful when handling blood from an infected dog, and any accidental needle sticks should be reported to the Centers for Disease Control and Prevention immediately.20
Lora R. Ballweber, DVM, MS
Department of Microbiology, Immunology, and Pathology
College of Veterinary Medicine and Biomedical Sciences
Colorado State University
Fort Collins, CO 80523
REFERENCES
1. Conboy GA. Canine angiostrongylosis: the French heartworm: an emerging threat in North America. Vet Parasitol 2011;176:382-389.
2. Koch J, Willesen JL. Canine pulmonary angiostrongylosis: an update. Vet J 2009;179:348-359.
3. Morgan ER, Shaw SE, Brennan SF, et al. Angiostrongylus vasorum: a real heartbreaker. Trends Parasitol 2005;21:49-51.
4. Bourque AC, Conboy G, Miller LM, et al. Angiostrongylus vasorum infection in 2 dogs from Newfoundland. Can Vet J 2002;43:876-879.
5. Conboy G. Natural infections of Crenosoma vulpis and Angiostrongylus vasorum in dogs in Atlantic Canada and their treatment with milbemycin oxime. Vet Rec 2004;155:16-18.
6. Helm J, Gilleard JS, Jackson M, et al. A case of canine Angiostrongylus vasorum in Scotland confirmed by PCR and sequence analysis. J Small Animal Pract 2009;50:255-259.
7. Patterson-Kane JC, Gibbons LM, Jefferies R, et al. Pneumonia from Angiostrongylus vasorum infection in a red panda (Ailurus fulgens fulgens). J Vet Diagn Invest 2009;21:270-273.
8. Chapman PS, Boag AD, Guitian J, et al. Angiostrongylus vasorum infection in 23 dogs (1999-2002). J Small Animal Pract 2004;45:435-440.
9. Sasanelli M, Paradies P, Otranto D, et al. Haemothorax associated with Angiostrongylus vasorum infection in a dog. J Small Animal Pract 2008;49:417-420.
10. McKown RD, Veatch JK, Fox LB. New locality record for Heterobilharzia americana. J Wildl Dis 1991;27:156-160.
11. Johnson EM. Canine schistosomiasis in North America: An underdiagnosed disease with an expanding distribution. Compend Contin Ed Pract Vet 2010;March:E1-E4.
12. Fabrick C, Bugbee A, Fosgate G. Clinical features and outcome of Heterobilharzia americana infection in dogs. J Vet Intern Med 2010;24:140-144.
13. Rohrer CR, Phillips LA, Ford SL, et al. Hypercalcemia in a dog: a challenging case. J Am Anim Hosp Assoc 2000;36:20-25.
14. Fradkin JM, Braniecki AM, Craig TM, et al. Elevated parathyroid hormone-related protein and hypercalcemia in two dogs with schistosomiasis. J Am Anim Hosp Assoc 2001;37:349-355.
15. Ruth J. Heterobilharzia americana infection and glomerulonephritis in a dog. J Am Anim Hosp Assoc 2010;46:203-208.
16. Reisenman CE, Lawrence G, Guerenstein PG, et al. Infection of kissing bugs with Trypanosoma cruzi, Tucson, Arizona, USA. Emerg Infect Dis 2010;16:400-405.
17. Dorn PL, Perniciaro L, Yabsley MJ, et al. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis 2007;13:605-607.
18. Leiby DA, Herron RM Jr, Garratty G, et al. Trypanosoma cruzi parasitemia in US blood donors with serologic evidence of infection. J Infect Dis 2008;198:609-613.
19. Kjos SA, Snowden KF, Craig TM, et al. Distribution and characterization of canine Chagas disease in Texas. Vet Parasitol 2008;152:249-256.
20. Barr SC. Canine Chagas' disease (American trypanosomiasis) in North America. Vet Clin North Am Small Anim Pract 2009;39:1055-1064.
21. Cardinal MV, Reithinger R, Gürtler RE. Use of an immunochromatographic dipstick test for rapid detection of Trypanosoma cruzi in sera from animal reservoir hosts. J Clin Microbiol 2006;44:3005-3007.
22. Nieto PD, Boughton R, Dorn PL, et al. Comparison of two immunochromatographic assays and the indirect immunofluorescence antibody test for diagnosis of Trypanosoma cruzi in dogs in south central Louisiana. Vet Parasitol 2009;165:214-247.
23. Rosypal AC, Hill R, Lewis S, et al. Evaluation of a rapid immunochromatographic dipstick test for detection of antibodies to Trypanosoma cruzi in dogs experimentally infected with isolates obtained from opossums (Didelphis virginiana), armadillos (Dasypus novemcinctus), and dogs (Canis familiaris) from the United States. J Parasitol 2011;97:140-143.