Feline atypical mycobacterial panniculitis: Treatment, monitoring, and prognosis
Feline nonhealing nodular or ulcerative dermatoses can present veterinary practitioners with diagnostic and therapeutic challenges.
FELINE NONHEALING nodular or ulcerative dermatoses can present veterinary practitioners with diagnostic and therapeutic challenges. These lesions can initially be mistaken for cat bite abscesses, but empirical abscess management consisting of drainage, cleansing, and short-term systemic antibiotic treatment routinely fails. Feline atypical mycobacterial panniculitis (AMP) (also called opportunistic mycobacterial granuloma) is one of many causes of chronic, draining, nodular skin and subcutaneous diseases in cats. Feline AMP results from infection with rapidly growing Runyon group IV mycobacteria. In the previous article, we reviewed the clinical and diagnostic features of AMP. This article focuses on the treatment options available in cats with AMP.
To initiate the best therapeutic plan, confirm an etiologic diagnosis of AMP by morphologically identifying the infecting mycobacteria in cytologic or histologic samples and by obtaining a positive, noncontaminated tissue culture. Performing antimicrobial susceptibility profiles on atypical mycobacterial isolates also facilitates successful treatment planning and implementation. Once a definitive diagnosis of AMP is established, practitioners and clients are faced with several treatment choices, including medical therapy only or a combination of medical and surgical therapies.
Medical treatment
The Runyon group IV mycobacteria that cause AMP include Mycobacterium smegmatis, Mycobacterium phlei, Mycobacterium fortuitum, and Mycobacterium chelonae. These agents are rapid-growing, nontuberculous mycobacteria that do not respond to antitubercular drugs. Ideally, selecting an antimicrobial drug is based on individual susceptibility profiles. Antibiotic susceptibilities vary among strains of mycobacteria. They also occasionally differ within strains in different geographic regions and can be influenced by previous and concurrent antibiotic treatment.1-3 For instance, multiple studies have demonstrated that strains of M. smegmatis are usually susceptible to more antibiotics than strains of M. fortuitum are, although both of these mycobacteria are often susceptible to fluoroquinolones, gentamicin, and doxycycline.1,4 Previous publications also report that M. fortuitum is more commonly isolated from North American cats with AMP, while M. smegmatis was the most prevalent strain seen in a survey of 49 Australian cats.1,5,6 Certain pathogenic mycobacteria, most notably Mycobacterium tuberculosis, have been known to rapidly develop antibiotic resistance during treatment, and increasingly resistant strains may be an emerging feature of atypical mycobacterial infections as well.1,7
No well-established guidelines allow a clinician to predict whether a cat with AMP will have an adequate clinical response to medical therapy or will eventually require medical and surgical therapy (Figure 1). Since many authors have reported cures with single-agent antimicrobial therapy1,7,8 and appropriate antimicrobial therapy is recommended before performing most surgical procedures in cats with AMP,1,7,8 we recommend that antibiotic administration be considered the first step in the comprehensive treatment of all cats with AMP.
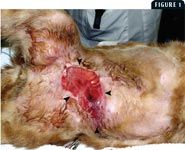
1. This cat was presented with a chronic lesion on the caudal ventral abdomen. The arrowheads outline the extent of the wound at presentation. The diagnosis was AMP, and the cat was treated for six weeks with appropriate antibiotics, followed by aggressive surgical excision.
After the susceptibility of the mycobacteria causing AMP is known, clinicians are often left with multiple drug choices, as most strains are susceptible to several classes of antimicrobials. The ideal antimicrobial agent should 1) be effective against the infecting strain, 2) be approved for use in cats, 3) have favorable pharmacologic and pharmacokinetic parameters in the intended species and disease for which it will be prescribed, and 4) have minimal or clinically acceptable side effects. Of the most commonly used agents for the treatment of AMP, fluoroquinolones have several advantages. Fluoroquinolones, such as enrofloxacin, are bactericidal, have good penetration into dermal and subcutaneous structures, and achieve adequate concentrations within phagocytic immune cells.1,9 Their use is well-tolerated in cats within the recommended oral dose range (Table 1). Retinal degeneration has recently been described in cats receiving long-term, extralabel dosages of enrofloxacin10 and has been documented when used to treat a cat with AMP,11 so be sure to inform owners of this possible complication when long-term therapy with enrofloxacin is anticipated. Another concern commonly voiced in human medicine about fluoroquinolone usage is the possibility for the rapid development of antimicrobial resistance,9 but currently there is insufficient clinical evidence suggesting that this is problematic in cats with AMP.

TABLE 1: Antimicrobials and Dosages Used to Treat Feline AMP
Doxycycline, a bacteriostatic tetracycline, has also been shown to be efficacious and safe for treating AMP (Table 1) and is a more economical choice than enrofloxacin. Routine doxycycline use may also reduce the risk of the development of resistant, mutant strains of mycobacteria.1 The potentially reduced risk is based on experience with people that indicates that doxycycline has low mutational resistance.1 It is also based on doxycycline's high lipid solubility as well as on other pharmacokinetic data on area-under-the-curve concentration. Vomiting and inappetence are potential dose-limiting adverse effects of doxycycline. Increasing a cat's doxycycline dosage is best achieved by a gradual escalation over two or three weeks.1 Doxycycline has been reported to cause esophageal strictures in cats, and oral therapy should be immediately stopped in any animal that develops signs of regurgitation or dysphagia.12 Doxycycline should be given with food or followed immediately by oral fluid.
Although studies in cats are limited, based on previous analyses of the general susceptibility profiles of multiple mycobacterial isolates1,3 and the previous discussion, administration of doxycycline, ciprofloxacin, or enrofloxacin alone or in combination has been recommended for AMP cases in which individual susceptibility data cannot be obtained. At this time, if susceptibility data are available, routine use of multiple concurrent antimicrobials to initially treat AMP offers no important advantages over monotherapy.1
Some agents that should not be considered for empirical use, as they can be associated with variable susceptibility profiles or potentially serious toxicities, do deserve mention as potential agents for treating AMP if bacterial culture and antimicrobial sensitivity results indicate that the mycobacterial isolate in a specific case is sensitive to these drugs. Most strains of M. smegmatis have reportedly been susceptible to potentiated sulfonamides, such as trimethoprim-sulfamethoxazole (Table 1), although one study reported that multiple isolates of M. fortuitum were uniformly resistant to trimethoprim.1 Some of the newer macrolide derivatives, including clarithromycin and azithromycin, have been useful for treating atypical mycobacterial infections in people and cats (Table 1).1,7 Although these macrolides have broad antimicrobial spectrums and prolonged pharmacologic effects, their susceptibility profiles are often unpredictable.1 Many mycobacterial species from cats with AMP will also be susceptible to aminoglycosides such as gentamicin (Table 1). However, routine aminoglycoside use can be hindered by poor penetration into adipose tissues, potential nephrotoxicity, and a requirement for parenteral administration.1,7
The optimal duration of therapy required to effectively treat AMP is unknown. Despite the fact that some cats with AMP have demonstrated lasting complete remissions after just a few weeks of antibiotic therapy1,8 and that spontaneous remissions from cutaneous, atypical mycobacterial infections occur frequently in people,7 current recommendations suggest that cats demonstrating an initial positive response to antibiotic monotherapy receive three to six months of continuous treatment.1 Another therapeutic regimen advocates that therapy be continued for one month beyond the resolution of all clinical signs.1 As some cases of AMP can be associated with disease that historically waxes and wanes, prolonged therapy is designed to eliminate clinically dormant organisms, and some cats require indefinite maintenance therapy to maintain remission.1,7,11 Bathing with antimicrobial shampoo and using Elizabethan collars and analgesics may improve a cat's appearance and quality of life, but they do not seem to substantially affect the disease's clinical course. Do not give affected cats immunosuppressive agents, such as corticosteroids.
Surgical treatment
Surgery is an often effective but primarily adjunctive (used when single modality treatment fails to resolve clinical disease) tool for feline AMP. Surgically excising affected tissues without administering appropriate antimicrobial therapy is doomed to failure. Preoperative and perioperative antimicrobials are advocated to reduce the microbial burden before surgery and to ensure eradication of any residual mycobacteria. Surgery should be considered and potentially incorporated into the therapeutic plan in cats with AMP if 1) the cutaneous disease burden is extensive, 2) one or more locally confined, large lesions are severe or hinder the patient's mobility, or 3) clinical progress has become static after initial improvement with medical treatment.
Chronic wounds in cats pose a formidable challenge to veterinary surgeons. These chronically affected or infected cats may have both nonhealing wounds and nodular or ulcerative lesions. In the veterinary literature, the role of surgery in treating chronic skin lesions in cats is controversial and depends on the nature and extent of the lesion. It is important to remember that chronic wounds in cats are associated with myriad infectious agents, such as mycobacteria, L-form bacteria, and feline cowpox, just to list a few. Other causes such as primary immunodeficiency, hyperadrenocorticism, neoplasia, foreign body reaction, and drug reaction must also be considered and ruled out before surgical intervention is considered.
The timing of surgery depends directly on the size and extent of the lesions. Patients with lesions that can be easily excised and closed are excellent candidates for early surgical intervention. The rationale for early surgical intervention is twofold: 1) to obtain adequate surgical margins and, therefore, serve as a possible curative procedure, and 2) to obtain adequate tissue samples for histologic examination, bacterial culture and antimicrobial sensitivity testing, and special staining procedures. It is vital to obtain adequate surgical margins in all dimensions. In our experience, in cases in which lesions are on the flank or abdomen, complete surgical excision must include removing body wall. We have found that less aggressive attempts at resection have poor results and usually necessitate a second, more aggressive surgery. We have found that in most cases, closing the body wall can be performed without serious complications (Figure 2). Lesions on the thorax may necessitate the removal of all underlying muscle and fascia to the level of the ribs.
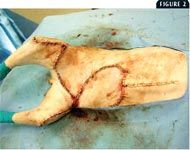
2. The immediate postoperative appearance of the cauÂdal ventral abdomen of a cat with a chronic drainÂÂing skin lesion due to AMP. AgÂgressive radical excision must be performed to remove all affected tissue.
Extensive lesions that would be difficult to close after surgery should first be treated medically. Multiple skin biopsy samples for histologic examination, bacterial culture and antimicrobial sensitivity testing, and special staining can be obtained at this time, followed by four to six weeks of appropriate antibiotic therapy. The patient should then be evaluated to determine whether surgical excision is possible. If surgical excision can be performed, wide surgical margins must be obtained as previously mentioned, and antibiotic therapy must be continued for a minimum of four weeks.
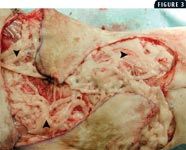
3. An intraoperative photo showing the use of omentum (arrowheads) to enhance healing in the cat shown in Figure 1.
If multiple lesions exist, surgical excision can be staged, with early surgical removal being performed on those lesions that can easily be excised and closed. Medical treatment is initiated for four to six weeks after you receive the results of histologic examination and bacterial culture and antimicrobial sensitivity testing. After this four to six weeks, the remaining wounds can be evaluated for surgical removal.
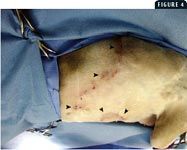
4. A cat with recurrence of lesions on the lateral abdomen and ventral abdomen six weeks after aggressive surgical excision (the cat head is to the left and the ventral aspect of the abdomen is toward the bottom of the photo). The arrowheads are pointing to the draining tracts. The cat had received an antibiotic postoperatively for a minimum of three weeks.
Some reports in the veterinary literature advocate omentoplasty to treat chronic wounds in cats (Figure 3).13-15 The functions of the omentum include fibrinolysis, tissue adhesion, angiogenesis, immune surveillance, and lymphatic drainage.13-18 In one retrospective study involving five cats with chronic skin lesions that were treated with omentoplasty, complete and uneventful healing occurred in all five cats.15 The mean follow-up time in the study was 2.5 years. Another retrospective study involving chronic axillary wounds in 10 cats that were treated with omentoplasty found that a long-term cure was achieved in seven cats.13 These reports suggest that in cats with chronic wounds, omentoplasty may be valuable and should be considered. Despite radical surgical excision of diseased tissue, recurrence in cats with AMP is high (Figures 4 & 5). Owners must be warned of the potential for recurrence before surgery. These surgeries typically require referral.
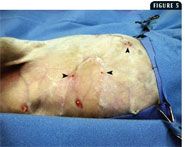
5. The same cat as in Figure 3 (the cats head is to the right of the photo). The lesions recurred as drainÂing tracts (arrowheads) despite aggressive surgical exÂciÂsion and use of a caudal superficial epigastric flap.
Therapeutic monitoring and prognosis
After initiating medical therapy, reevaluate cats with a clinical examination once or twice a month. In patients with improving lesions, continue the current treatment and monitoring protocol for three to six months, or one or two months beyond the resolution of all lesions. Cats that experience initial, but incomplete clinical improvement that later plateaus should be examined on a biweekly basis. If lesion resolution is not appreciated over an additional four-week period, consider adjunctive surgical débridement of the affected areas and continue antimicrobial monotherapy.1,7 In cats with AMP that suffer from progressive cutaneous disease despite treatment with an appropriate antimicrobial, perform additional tissue biopsies and cultures with antimicrobial sensitivity testing to confirm the diagnosis and document the development of potential antimicrobial resistance. In our experience, these difficult cases are best treated with both surgery and long-term, combination antimicrobial treatment based on the susceptibility patterns obtained from the most recent biopsy. The overall prognosis for cats with AMP remains guarded.
Summary
Because of medical and surgical advances that allow for an earlier and more accurate diagnosis and safer, more aggressive treatment, feline AMP should be considered a manageable and potentially curable disease.1,7 Consistent and thorough patient monitoring and client communication are paramount to successful long-term outcomes. The chronic nature of this disease requires that the owner of a cat with AMP be adequately prepared for the time, potential disappointment, and financial commitments associated with treatment. Unsuccessful treatment or no treatment results in disease progression, debilitation, pain, and death or euthanasia.
Thomas O. Manning, DVM, MS, DACVD
John H. Rossmeisl Jr., DVM, MS, DACVIM (internal medicine and neurology)
Otto I. Lanz, DVM, DACVS
Department of Small Animal Clinical Sciences
Virginia-Maryland Regional College of Veterinary Medicine
Virginia Tech
Blacksburg, VA 24061
REFERENCES
1. Malik, R. et al.: Infection of the subcutis and skin of cats with rapidly growing mycobacteria: A review of microbiological and clinical findings. J. Feline Med. Surg. 2 (1):35-48; 2000.
2. Jang, S.S.; Hirsch, D.C.: Rapidly growing members of the genus Mycobacterium affecting dogs and cats. JAAHA 38 (3):217-220; 2002.
3. Koontz, F.P. et al.: Etest for routine clinical antimicrobial susceptibility testing of rapid-growing mycobacteria isolates. Diagn. Microbiol. Infect. Dis. 19 (3):183-186; 1994.
4. Wallace, R.J. Jr.: Recent changes in taxonomy and disease manifestations of the rapidly growing mycobacteria. Eur. J. Clin. Microbiol. Infect. Dis. 13 (11):953-960; 1994.
5. Kunkle, G.A. et al.: Rapidly-growing mycobacteria as a cause of cutaneous granulomas: Report of five cases. JAAHA 19:513-521; 1983.
6. White, S.D. et al.: Cutaneous atypical mycobacteriosis in cats. JAVMA 182 (11):1218-1222; 1983.
7. Lemarie, S.L.: Mycobacterial dermatitis. Vet. Clin. North Am. (Small Anim. Pract.) 29 (6):1291-1301; 1999.
8. Studdert, V.P.; Hughes, K.L.: Treatment of opportunistic mycobacterial infections with enrofloxacin in cats. JAVMA 201 (9):1388-1390; 1992.
9. Hooper, D.C.; Wolfson, J.S.: Fluoroquinolone antimicrobial agents. N. Engl. J. Med. 324 (6):384-394; 1991.
10. Gelatt, K.N. et al.: Enrofloxacin-associated retinal degeneration in cats. Vet. Ophthalmol. 4 (2):99-106; 2001.
11. Alander-Damsten, Y.K. et al.: Panniculitis, due to Mycobacterium smegmatis, in two Finnish cats. J. Feline Med. Surg. 5 (1):19-26; 2003.
12. McGrotty, Y.L.; Knottenbelt, C.M.: Oesophageal stricture in a cat due to oral administration of tetracyclines. J. Small Anim. Pract. 43 (5):221-223; 2002.
13. Lascelles, B.D. et al.: Use of omental pedicle grafts in the management of non-healing axillary wounds in 10 cats. J. Small Anim. Pract. 39 (10):475-480; 1998.
14. Lascelles, B.D.; White, R.A.: Combined omental pedicle grafts and thoracodorsal axial pattern flaps for the reconstruction of chronic, nonhealing axillary wounds in cats. Vet. Surg. 30 (4):380-385; 2001.
15. Brockman, D.J. et al.: Omentum-enhanced reconstruction of chronic nonhealing wounds in cats: Techniques and clinical use. Vet. Surg. 25 (2):99-104; 1996.
16. Bray, J.P. et al.: Partial resection and omentalization: A new technique for management of prostatic retention cysts in dogs. Vet. Surg. 26 (3):202-209; 1997.
17. Lafond, E. et al.: Omentalization of the thorax for treatment of idiopathic chylothorax with constrictive pleuritis in a cat. JAAHA 38 (1):74-78; 2002.
18. White, R.A.; Williams, J.M.: Intracapsular prostatic omentalization: A new technique for management of prostatic abscesses in dogs. Vet. Surg. 24 (5):390-395; 1995.