Feline panleukopenia: A diagnostic laboratory's perspective
Feline panleukopenia is a highly contagious disease with a worldwide distribution in wild and domestic felids.
FELINE PANLEUKOPENIA (FPL) is a highly contagious disease with a worldwide distribution in wild and domestic felids. It has been officially recorded in cat populations since the early 20th century but has likely existed for hundreds of years.1 Settings such as farms, animal shelters, pet shops, kennels, and research colonies are ideal for the spread and transmission of FPL. Practitioners working with sick cats from these populations and with stray cats that have unknown contact and vaccination histories should have a good working knowledge of the pathogenesis of FPL and how to diagnose it. They must also be diligent in using procedures and precautions that will minimize the risk of infecting their facilities and spreading the disease among its inhabitants. Diagnostic laboratories frequently perform necropsies on and receive samples from cats suspected of having FPL. In this article, we review the necropsy findings and histopathologic lesions of FPL and discuss which diagnostic tools are available as well as preventive measures.
ETIOLOGIC AGENT
FPL virus is a member of the family Parvoviridae, a group of small, nonenveloped, single-stranded DNA viruses. Other members of this family include mink enteritis virus and canine parvovirus. Canine parvovirus is thought to have emerged from FPL virus in the late 1970s,2,3 and while this original strain of canine parvovirus (canine parvovirus type 2 [CPV2]) could not infect cats, more recently emerged isolates of canine parvovirus, CPV2a and CPV2b, can.3,4 All of these viruses infect rapidly dividing cells in the intestinal tract and cause marked enteritis. Parvoviruses do not make their own DNA polymerase, so replication occurs in the nucleus, and the initial step of DNA replication depends on the DNA polymerase expressed in infected cells during mitosis. Intranuclear inclusion bodies, observable by light microscopy, provide visible evidence of viral replication.
PATHOPHYSIOLOGY AND GROSS AND HISTOPATHOLOGIC LESIONS
During the acute phase of the infection, FPL virus particles may be shed in saliva, vomit, feces, or urine.1,5 Virus particles can be transmitted to a susceptible host, usually by an oronasal route, either through direct contact with an infected cat or by fomites. The virus initially infects and replicates in oropharyngeal lymphoid tissue.6 It then disseminates to other tissues between two and seven days7 and is present in Peyer's patches in the intestine by three or four days after infection.8 Cell replication is required for parvoviral DNA synthesis, so rapidly dividing cells are the preferred sites of replication. Thus, in cats with FPL, intestinal crypt epithelium, bone marrow progenitor cells, and lymphoid organs are most severely affected. Mitotic rates are lower in the colon than they are in the small intestine, and lesions are also milder in the large intestine.7 Mitotic rates in the cerebella and retinas of neonates are increased, making these organs primary sites affected in neonatal kittens.7
Cats of all ages can develop FPL, but kittens and young cats are most commonly affected. In kittens, the period of greatest susceptibility to infection is when maternal antibodies are waning and vaccine-induced immunity has not yet fully developed. Cases tend to cluster when there is a buildup of susceptible kittens, a function of the seasonal breeding in a given cat population.1 FPL is associated with high morbidity and mortality, but infections can range from subclinical to severe depending on the immune status and age of a cat when it is infected. Infection in pregnant queens can result in fetal resorption, mummification, abortion, or stillbirth.6 Fetuses infected in utero that survive and kittens less than a few weeks of age that become infected can have cerebellar hypoplasia, retinal dysplasia, and optic neuropathy.7
Peracute enteritis is a manifestation of the disease most often seen in kittens less than 16 weeks of age. In these cases, there is severe intestinal damage, and death can occur within 24 hours because of a combination of secondary bacterial septicemia and endotoxemia in the face of profound leukopenia.6 Kittens more than 16 weeks of age tend to have less severe disease; in these cases, supportive treatment that addresses severe dehydration and intestinal bacterial invasion can be effective. While there is no question that FPL virus infects and kills dividing cells, some evidence suggests that secondary bacterial infections make an equal or greater contribution to the clinical signs and the intestinal damage in cats with FPL. This important component of the pathogenesis of FPL has been illustrated in studies in which specific pathogen-free cats infected with FPL virus developed mild or no intestinal lesions.4,9 Adult cats are generally the least severely affected and may show mild signs or none at all.
The greatest fecal virus shedding corresponds with the peak of clinical disease, but keep in mind that the virus can be shed for up to six weeks after recovery7 and that subclinical animals can also shed virus. Lifelong immunity is thought to follow recovery from disease, and a carrier state of the disease has never been identified. So persistence of the disease in cat colonies is attributable to persistence of the virus in the environment and shedding from new infections, not chronic shedding.
Clinical signs of FPL include depression, abdominal pain, vomiting, diarrhea that may be bloody, and fever. Owners often report finding their cats with their heads hanging over their water dishes.6 The fevers are diphasic, peaking at 24 hours and again at 48 hours.10 Clinical laboratory findings include neutropenia, often with lymphopenia and anemia, and electrolyte and total protein concentrations that reflect dehydration, vomiting, and diarrhea.6
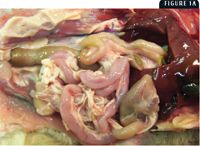
1A. Gross lesions of FPL virus infection. Figure 1A shows a flaccid small intestine from a 3-month-old kitten.
At necropsy, there may be nasal and ocular exudation and tacky mucous membranes. A consistent and particularly striking finding in cases of FPL is flaccidity of the intestine, often with mucosal sloughing and hemorrhage (Figures 1A & 1B). Normal feline small intestine is turgid and slightly coiled. A ground-glass appearance to the intestinal serosa, lymph node enlargement and edema, and a gelatinous, greasy, or liquid appearance to the bone marrow may also be present.10 Histologic examination of the intestine reveals dilated crypts lined by irregular, hyperplastic epithelial cells; fusion and blunting of the villi; and epithelial necrosis. In some cases, eosinophilic intranuclear inclusions are identifiable in infected crypt epithelium (Figure 2). In lymphoid tissues, edema, hyperemia, and lymphoid necrosis are seen frequently, and occasional intranuclear inclusion bodies are found in histiocytes.10
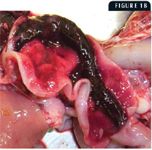
Figure 1B shows a hemorrhagic and flaccid small intestine from an 8-month-old kitten. These cases are from two separate FPL outbreaks that occurred in animal shelters in Wisconsin in 2003.
DIAGNOSTIC TESTS
FPL can be presumptively diagnosed based on a consistent history, clinical signs, and clinical pathology findings. FPL can also be presumptively diagnosed postmortem based on the gross and histopathologic lesions discussed above. However, there are many other FPL diagnostic methods.
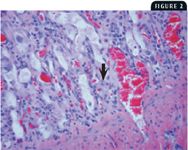
2. This photomicrograph from the small intestine of a cat with FPL shows dilatation of the intestinal crypts, hyperplasia and necrosis of the crypt epithelium, and a viral intranuclear body in an infected epithelial cell (arrow) (40X).
Antibody detection
Two serologic tests, serum neutralization and hemagglutination inhibition, can be helpful in determining the activity of FPL in a feline population. Both tests should be performed on paired sera to determine whether there is a rise in titer between the acute and convalescent samples.7 Both sera should be submitted to the same laboratory and should be assayed in the same test run to obtain reliable data.
Virus detection
Fecal antigen test kits such as the SNAP Parvo Antigen Test (Idexx) are only approved and licensed for detecting canine parvovirus, but it is generally known that they also detect FPL viral antigen in feline feces.6,11 These tests are used extralabel because they allow rapid, inexpensive, in-house detection of the virus. They are sensitive and specific but can detect FPL antigen in cats vaccinated with a modified live FPL vaccine within five to 12 days. Another type of bench-top test, a one-step immunochromatographic test not currently marketed in the United States, has been reported with a similar sensitivity to the SNAP Parvo Antigen Test and was developed for use with feline, canine, and mink fecal samples.11
FPL virus can be detected with a transmission electron microscope (TEM) by using fecal or intestinal tissue samples. This method can be used to confirm clinical cases or necropsy cases when classic gross and histopathologic lesions are not identified. Tissue samples are scraped and pulverized to release virus particles from cells. The particles are concentrated by ultracentrifugation, treated with 4% phosphotungstic acid, and then aerosolized onto a carbon-coated grid. The grid is placed in the TEM and screened for viral particle morphology (Figure 3). This form of microscopy has the advantage of enabling screening for more than one type of virus at a time. It is a good method for FPL virus detection because it can be done on fecal material, is a technique offered by most diagnostic laboratories, and allows the detection of all strains of parvovirus, including CPV2a and CPV2b. A drawback to the test is a relatively low sensitivity. The concentration of viral particles must be in the 106 to 107 range to be detected,12 and in fecal sample submissions the concentration of virus depends on the level of shedding of the virus at the time the samples are collected. Maximal virus shedding corresponds with the onset of diarrhea, so sending fresh fecal samples from this phase in the disease can increase the sensitivity of the test.

3. An electron micrograph of parvovirus particles measuring 20 to 24 nm.
Several conventional polymerase chain reaction (PCR) tests have a sensitivity level of 103 viral particles per gram of raw feces.13 A nested set PCR assay has also been developed that can detect between 101 and 102 viral particles.14 These assays have been standardized for detection in feces and are not affected by PCR inhibitors in feces. In addition, methods that use formalin-fixed tissue have also been reported3,15,16 for use with postmortem samples. Using PCR tests requires considerable expertise. While they are highly sensitive, they are also easily contaminated; this is especially true with nested set assays.
Immunofluorescent antibody staining (IFA) is another useful diagnostic tool, which detects FPL viral antigens in cryostat tissue sections. The preferred tissue for this technique is fresh intestine, but spleen and thymus may also be used. A specific mouse monoclonal antibody directed against FPL virus proteins is applied to the cryosections and incubated in a humidified chamber. After unbound monoclonal antibody is washed away, fluorescein isothiocyanate conjugated to anti-mouse secondary antibody is added to the section, followed by incubation and a wash cycle. Epifluorescence microscopy is then used to visualize FPL-specific, apple-green fluorescence. Because the virus replicates in the rapidly dividing epithelial cells of the mucosal crypts, these sites appear as localized areas of specific fluorescence (Figure 4). The IFA procedure is used when a rapid, sensitive, and specific test is required. However, the results depend on tissue samples, and the tissue quality greatly affects results. Highly autolyzed or degraded tissues rarely yield definitive staining.
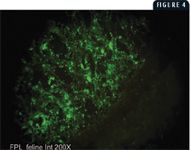
4. Epifluorescence microscopy of small intestinal mucosa from a cat heavily infected with FPL virus. Note the concentration of fluorescent signal in the mucosa and lack of fluorescence in the smooth muscle wall (lower right) (20X).
Finally, virus isolation for FPL virus has been successful in feline cell lines that are young and rapidly dividing. A commonly used cell line is Crandall feline kidney cells. FPL virus is disseminated throughout the body within seven days and can be recovered from feces and urine for several weeks after infection,7 but many laboratories will not accept fecal or urine samples for virus isolation because these samples can be toxic to cells used for culture. Although not commonly done, virus can also be cultured from oropharyngeal swabs and buffy coat samples. It is critical that these samples be obtained during the early viremic phase, submitted in viral transport media, and chilled en route. Some suppliers of specialized viral culture media include Becton, Dickinson and Company (Franklin Lakes, N.J.) and Remel (Lenexa, Kan.). Appropriate postmortem samples include spleen, mesenteric lymph nodes, and ileum. These samples should be submitted chilled by overnight delivery. In most laboratories, virus cultures are incubated for seven days with one blind passage, resulting in an average turnaround of two weeks. If cytopathic effect is observed, the cells will be stained with FPL-specific conjugate to confirm the presence of the virus. Virus isolation is useful to obtain an isolate for further characterization, particularly in epidemiology studies. One of the principal drawbacks of virus isolation is the length of time between submission and receipt of results.
PREVENTION AND CONTROL
Parvoviruses are remarkably stable in the environment, and because these viruses are also resistant to lipid solvents and disinfectants, they are among the toughest viral particles to eradicate from a contaminated facility. A gram of infected stool from a clinical case of FPL can contain as much as 109 viral particles.17 Reports on the exact longevity of the virus are variable, and many environmental factors affect the duration of virus viability. However, it is generally accepted that parvovirus particles can infect new hosts for six months to a year after they are shed,1,7 and there are many anecdotal reports of cases of FPL occurring on premises years after infected cats have recovered or been removed.
Cats with FPL can require intense supportive care, but hospitalization of highly contagious animals puts other patients at risk. When treating patients suspected of having FPL, a quarantine facility and diligent staff able to follow strict decontamination procedures are necessary to maintain an environment free of infectious particles. Parvovirus particles spread easily on hands and on shoes, clothing, and other fomites. So changing clothes, regular hand washing, and the use of foot baths are basic quarantine procedures that should be instituted. FPL virus can be inactivated by using a 1:20 to 1:32 dilution of sodium hypochlorite (4 oz household bleach in 1 gal water); 4% formalin solutions and 1% glutaraldehyde solutions also inactivate the virus.6,18,19 However, because of the robust nature of the virus, sanitation measures alone cannot be relied on to prevent the spread of this disease.
Vaccination is inexpensive, confers long immunity, and is the most effective measure for preventing FPL. Modified live and killed vaccines are available, which are often combined with feline calicivirus and feline herpesvirus; both confer excellent immunity. A 1999 study showed that cats vaccinated with a single dose of an inactivated vaccine for FPL had solid protection for 7.5 years (the length of the study).20 The modified live vaccines will produce more rapid immunity than the killed vaccines, but they need to be used with caution because they can cause cerebellar hypoplasia in fetuses if given to pregnant queens or in kittens less than 4 weeks of age.10 The age during which maternal immunity blocks vaccine-induced immunity varies, but it can be as long as 14 to 16 weeks of age. Further, there is an unavoidable period of waning maternal immunity that is no longer protective but is still able to block vaccine-induced immunity. This small window of incomplete protection is the Achilles' heel of any vaccination regimen. Being able to convey these key concepts to owners can be critical in ensuring that kittens receive the appropriate number of booster vaccinations. Vaccination regimens for kittens vary with the product being used, but they typically start at 8 to 10 weeks of age, are repeated every two to four weeks, and conclude with a booster at 12 to 16 weeks of age.
CONCLUSION
FPL is often described as a fully preventable disease. But despite the availability of inexpensive vaccines that confer long immunity and an in-depth understanding of the pathogenesis of this disease, it remains one of the most common infectious feline diseases diagnosed at the Wisconsin Veterinary Diagnostic Laboratory and continues to be a problem in cat populations throughout the country. Practitioner awareness of FPL will allow efficient diagnosis of the disease and enable effective implementation of treatment and strategies to control outbreaks. Further, there is growing concern that an upsurge in feline diseases currently kept at bay by effective vaccines could follow the trend toward reduced vaccination in cats. While reducing FPL vaccination from the traditional annual regimen in adult cats is reasonable, decreasing the number of FPL vaccinations given to kittens would be extremely detrimental. FPL virus is both hardy and ubiquitous, and client education that expresses the importance of repeated vaccination in kittens to ensure development of immunity has become an even more important part of client education in the face of changing vaccination strategies.
Alexandra I. Brower, DVM, DACVP
Department of Pathobiological Sciences
School of Veterinary Medicine
Wisconsin Veterinary Diagnostic Laboratory
University of Wisconsin
Madison, WI 53706
Craig Radi
Dave Kruefer, BS
Wisconsin Veterinary Diagnostic Laboratory
University of Wisconsin
Madison, WI 53706
Kathy Toohey-Kurth, MS, PhD
Department of Pathobiological Sciences
School of Veterinary Medicine
Wisconsin Veterinary Diagnostic Laboratory
University of Wisconsin
Madison, WI 53706
REFERENCES
1. Timoney, J.F. et al.: The Parvoviridae. Hagan and Brunner's Microbiology and Infectious Diseases of Domestic Animals. Comstock Publishing Assoc., Ithaca, N.Y., 1988; pp 501-521.
2. Parrish, C.R.: Host range relationships and the evolution of canine parvovirus. Vet. Microbiol. 69 (1-2):29-40; 1999.
3. Truyen, U. et al.: Evolution of the canine parvovirus involved loss and gain of feline host range. Virology 215 (2):186-189; 1995.
4. Ikeda, Y. et al.: Feline host range of canine parvovirus: Recent emergence of new antigenic types in cats. Emerg. Infect. Dis. 8 (4):341-346; 2002.
5. Kahn, D.E.: Pathogenesis of feline panleukopenia. JAVMA 173 (5 Pt 2):628-630; 1978.
6. Wolf, A.M.: Other feline viral diseases. Textbook of Veterinary Internal Medicine, 5th Ed. (S.J. Ettinger; E.C. Feldman, eds.). W.B. Saunders, Philadelphia, Pa., 2000; pp 444-446.
7. Greene, C.E.: Feline panleukopenia. Infectious Diseases of the Dog and Cat, 2nd Ed. (C.E. Greene, ed.). W.B. Saunders, Philadelphia, Pa., 1998; pp 52-57.
8. Barker, I.K. et al.: The alimentary system. Pathology of Domestic Animals, Vol. 2, 4th Ed. (K.V.F. Jubb et al., eds.). Academic Press, San Diego, Calif., 1993; pp 193-198.
9. Carlson, J.H. et al.: Feline panleukopenia. I. Pathogenesis in germfree and specific pathogen-free cats. Vet. Pathol. 14 (1):79-88; 1977.
10. Jones, T.C. et al.: Veterinary Pathology, 6th Ed. Lea & Febiger, Philadelphia, Pa., 1997; pp 257-261.
11. Esfandiari, J.; Klingeborn, B.: A comparative study of a new rapid and one-step test for the detection of parvovirus in faeces from dogs, cats and mink. J. Vet. Med. B 47 (2):145-153; 2000.
12. Doane, F.W.; Anderson, N.: Methods for preparing specimens for electron microscopy. Electron Microscopy in Diagnostic Virology. Cambridge University Press, New York, N.Y., 1987; pp 14-31.
13. Schunck, B. et al.: A simple touch-down polymerase chain reaction for the detection of canine parvovirus and feline panleukopenia virus in feces. J. Virol. Methods 55 (3):427-433; 1995.
14. Hirasawa, T. et al.: Sensitive detection of canine parvovirus DNA by the nested polymerase chain reaction. Vet. Microbiol. 41 (1-2):135-145; 1994.
15. Meurs, K.M. et al.: Molecular screening by polymerase chain reaction detects panleukopenia virus DNA in formalin-fixed hearts from cats with idiopathic cardiomyopathy and myocarditis. Cardiovasc. Pathol. 9 (2):119-126; 2000.
16. Schatzberg, S.J. et al.: Polymerase chain reaction (PCR) amplification of parvoviral DNA from the brains of dogs and cats with cerebellar hypoplasia. J. Vet. Intern. Med. 17 (4):538-544; 2003.
17. Quinn, P.J. et al.: Parvoviridae. Veterinary Microbiology and Microbial Disease, 1st Ed. Blackwell Science Ltd., Iowa State Press, Ames, 2002; pp 346-350.
18. Kennedy, M.A. et al.: Virucidal efficacy of the newer quaternary ammonium compounds. JAAHA 31 (3):254-258; 1995.
19. Scott, F.W.: Virucidal disinfectants and feline viruses. AJVR 41 (3):410-414; 1980.
20. Scott, F.W.; Geissinger, C.M.: Long-term immunity in cats vaccinated with an inactivated trivalent vaccine. AJVR 60 (6):652-658; 1999.
UN, WHO address public health concern over avian flu transmission to humans
April 18th 2024Veterinary professionals working with certain animals are advised to take precautionary steps to minimize risk of infection, while researchers in Texas study potential H5N1 vaccines, antivirals, and antibody therapies for humans
Read More