Getting the vapors: An education in inhalation anesthetics
Once you know the basics of vapor pressure, solubility and minimum alveolar concentration, youll be able to compare inhalants speed, control and potencyand know if youre getting the straight story from that sales rep.
SasaStock / stock.adobe.com (equipment photo courtesy of AM Bickford)
Inhalational anesthetics have an air of mystery about them-an attribute that Mike Barletta, DVM, MS, PhD, DACVAA, finds amusing. “It just blows my mind that we don't really know how inhalants work,” he says with a laugh. “They're the most used anesthetic drugs, and we don't even know their full mechanism of action.”
But what is known can help you compare inhalants, says Dr. Barletta, associate professor of anesthesia at the University of Georgia, College of Veterinary Medicine. At a recent Fetch dvm360 conference, he described the following scenario: A representative for inhalant X comes to your clinic and says, “You need to buy inhalant X. It's way better than the inhalant Y you've been using.” If you know the basics regarding vapor pressure, solubility and minimum alveolar concentration (MAC), says Dr. Barletta, you'll be able to discern if X really is better than Y and worth the switch (and the potentially higher price tag).
Knowledge is power. To get the confidence, you'll need to understand these concepts-namely, vapor pressure, solubility and MAC.
What's so sensational about inhalational?
Inhalational anesthesia tops injectable in several ways, says Dr. Barletta. First, it allows you far more control, since you can adjust it throughout the anesthetic event.
It's also easier to monitor how much drug is in the animal. “If you have a gas analyzer for inhalants, you have a good idea of how much of the inhalant is in the animal's body every time it exhales,” he explains. “If you want to do that with injectable, you'd have to get a sample of the target organ, which in anesthesia is the brain. So you could do that-but it would probably be the last thing you do for that patient.”
Another perk: Injectable anesthetics are metabolized and renally excreted, while inhalational anesthetics are mostly eliminated via the lungs.
What's not so sensational about inhalational?
So why not use inhalants all the time? Anesthesia machines are expensive, bulky and require maintenance, says Dr. Barletta. Moreover, because inhalational anesthesia causes cardiovascular depression, it isn't well tolerated by all patients.
“I've had cases where I've had to turn the gas off and put the patient on injectable drugs because every time we'd turn on even minimal inhalants, the patient's blood pressure would drop to the floor,” he says.
Vapor pressure
“First things first,” says Dr. Barletta. “We talk about ‘gas anesthesia,' but we should be talking about ‘vapor anesthesia.' Isoflurane, sevoflurane and desflurane are administered as vapors. A vapor is a substance in its gaseous phase that can be condensed to a liquid by increasing the pressure without reducing the temperature.”
According to Dr. Barletta, this is possible because room temperature is lower than the critical temperature (i.e. the temperature above which the gas cannot be liquefied by pressure alone) of that specific vapor.
Vapor pressure. Defining vapor pressure requires a quick chemistry lesson. When molecules of a liquid evaporate in a closed container, they can't escape and eventually condense back into a liquid. When the rate of evaporation equals the rate of condensation, the gas is at equilibrium with the liquid, and the pressure it exerts at this point is defined as its vapor pressure. The temperature at which the vapor pressure equals the barometric pressure is termed the boiling point.
To put things into a practical perspective, Dr. Barletta looked up the atmospheric pressure for Kansas City, Missouri, where his session was taking place. “It's 740 mmHg at 20 C,” he notes. “The vapor pressure of desflurane is close to 700 mmHg at 20 C. That means that if you're going to open that bottle, it will start boiling at around 70 F. That's why desflurane has to be sealed in a bottle at all times. It has to be pressurized and locked into a special vaporizer before opening, which is why most people don't use desflurane.”
Table 1 (below) shows the boiling point, vapor pressure and maximum concentration of isoflurane, sevoflurane and desflurane.
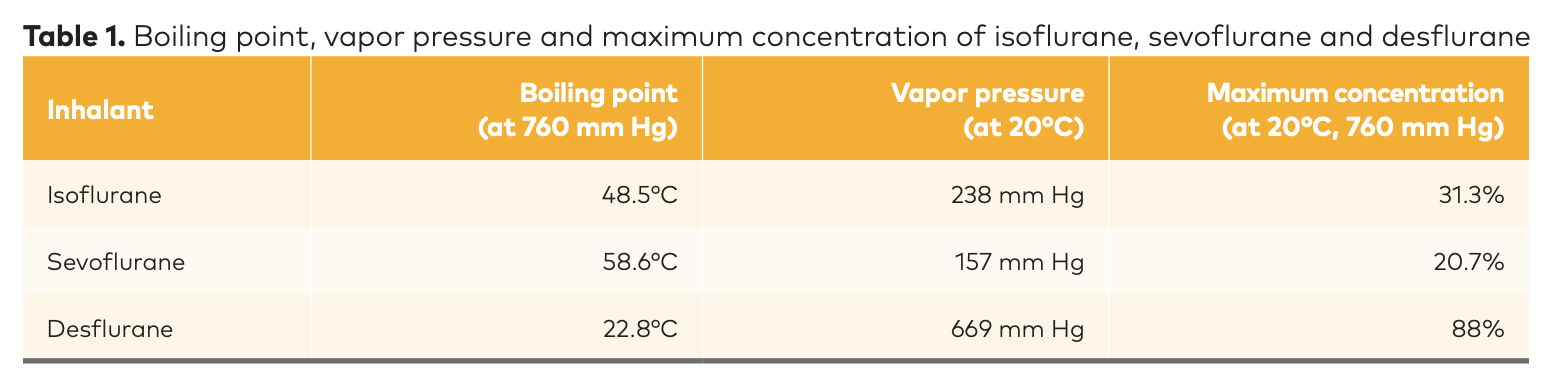
“Looking at the chart, you realize how much damage we can do if we're just putting some isoflurane on a cotton ball and putting it in front of a bird or cat or even a big dog,” says Dr. Barletta. “You're delivering about 32% isoflurane. How high does the dial go on your isoflurane vaporizer? Five percent. You can kill a dog if you leave the vaporizer at 5%, so imagine if you're giving almost 32%.”
Partial pressure. Because inhalants are delivered with other gases, it's important to understand partial pressure-the pressure exerted by a particular gas within a mixture-as well, says Dr. Barletta. “We talk about percentages, because inhalants are measured in volume percent,” he adds. “If I say I'm giving 2% isoflurane, what does that mean? It's the percent of the mixture that I'm giving. So if I give 2% of isoflurane, it means that maybe 95% is oxygen and the rest is all the other gases that are coming out of the animal.”
Here's the equation: Volume % = vapor pressure/barometric pressure x 100
“So if I want to calculate 2% isoflurane into mmHg, which is how we measure an inhalant's partial pressure, it's just 2% of the atmospheric pressure,” Dr. Barletta explains. So for Kansas City, it would be .02 x 740 mmHg = 14.8 mmHg.
Partial pressure plays a pivotal role in inhalational anesthesia, says Dr. Barletta. “Though we don't know much about inhalant anesthesia's mechanism of action, we do know that inhalants move thanks to the partial pressure gradient,” he says. “Gases move from areas of high partial pressure to areas of lower partial pressure. So when you turn on the anesthesia machine, which has a high partial pressure, the gas will move into the lungs, where the partial pressure is very low. Once the partial pressure gets high enough in the lungs, it moves to the blood, and from the blood to the organs. Movement continues until you reach equilibrium.”
When you turn off the gas, the process is reversed until the inhalant is completely eliminated and the patient wakes up.
Think of it this way, says Dr. Barletta: “Imagine I have two boxes with two different substances: air and blood. Inhalant molecules can move from one box to the other, but blood and air remain in their respective boxes. I pour inhalants into the air compartment. I have a certain amount of molecules, which is the concentration. These molecules start moving around in the air box and develop partial pressure. Because gases move from areas of high partial pressure to areas of lower partial pressure, inhalant molecules will start moving into the blood box until the partial pressures in each box are exactly the same.”
How many molecules are in each box? “We don't know. It depends on how well the molecules dissolve into the substances. I could have a lot of molecules in the air and very few in the blood and still have the same partial pressure in each, or vice versa,” Dr. Barletta explains. “But what I'm interested in is partial pressure because that's what I can measure. It's an important concept because in order to anesthetize an animal, I have to reach a certain partial pressure in its brain. At equilibrium, the partial pressure of inhalants in the animal's brain is the same as in its blood and lungs and what comes out of its lungs when it exhales. If I can measure that, I know exactly how much of the inhalant is in the animal's brain, which determines whether or not that animal is going to fall and stay asleep.”
Help your patients take a big breath
Because general anesthesia causes respiratory depression, Dr. Barletta recommends giving patients under gas anesthesia that aren't on a ventilator at least a breath every other minute. “It doesn't have to be constant,” he explains. “These animals can breathe on their own, but giving them a nice breath will open up the alveoli and prevent their lungs from collapsing-especially animals that are on the table for hours.”
This provides feedback as well. “The bag is your patient's lungs,” says Dr. Barletta. “For example, you might notice that the bag feels stiffer and you can't give a nice breath like you could a minute ago.”
Another perk: You're able to deliver a big breath of oxygen and inhalants, preventing the animal from waking up due to shallow breaths.
Solubility
“When we talk about an inhalant's solubility, we're referring to its solubility in blood,” says Dr. Barletta. “The solubility of inhalational anesthetics in blood is measured using the blood-gas partition coefficient, which describes how the gas partitions itself between the blood and the alveoli. The higher the coefficient, the more soluble the anesthetic is in the blood. An inhalant with a higher blood-gas partition coefficient will take longer to reach equilibrium (i.e. partial pressure) because it likes to sit in the blood. It's comfortable there. But an inhalant with a lower coefficient doesn't like it. It goes into the blood and bounces out and reaches equilibrium quickly.”
Thus, highly soluble anesthetic agents will take longer to induce general anesthesia, to recover and to make a change in anesthetic depth. “In other words,” he explains, “solubility means speed and control.”
As a professor, Dr. Barletta is always ready with an illustration: “Imagine I'm holding a cup of water over a sponge that's placed over a bucket. The water in the cup represents my inhalant, the sponge is the patient's blood and the bucket is the patient's brain. If I use the cup to pour a highly soluble inhalant into the sponge (i.e. blood), I'm going to have to pour for a long time before the sponge gets saturated enough for the inhalant to start leaking out of the sponge and into the bucket (i.e. brain) to reach the right partial pressure for my patient to fall asleep.”
For a highly insoluble inhalant, just switch out the sponge for a laminated sheet of paper. “As soon as I pour, it goes right over the edges of the sheet and into the bucket. Partial pressure is reached really fast, and the animal goes to sleep,” he says.
The blood-gas partition coefficients of isoflurane, sevoflurane and desflurane at 37 C are 1.4, 0.6 and 0.4, respectively. “Clinically speaking, there isn't a huge difference between the solubility of isoflurane and sevoflurane,” says Dr. Barletta. “So if someone tries to sell you on sevoflurane being faster, it's true, but not by that much-maybe a few minutes. Is it worth the extra money? I'll let you answer that question.”
Quick quiz on solubility
Dr. Barletta gave session attendees the following quiz to check their understanding:
If inhalant A is highly soluble and inhalant B has low solubility, which of the following is true?
1. Inhalant A will cause a faster induction.
2. Inhalant B will cause a faster induction.
3. Both will be equally fast.
Answer: 2. Because inhalant B doesn't mix well with the blood, it will reach partial pressure faster and will thus cause a faster induction.
MAC
If solubility can be boiled down to speed and control, then the MAC is all about potency. “The MAC is the volume percentage of an inhalation anesthetic required to prevent movement in 50% of subjects exposed to a supramaximal stimulus,” Dr. Barletta explains. “The lower the MAC, the greater the potency.”
It's also important to know that MAC values are additive, meaning two inhalants used in the same patient work synergistically, Dr. Barletta says.
MAC is species specific, so Table 2 (below) shows MAC values for isoflurane, sevoflurane and desflurane in cats and dogs. The lower the MAC, the higher the potency.
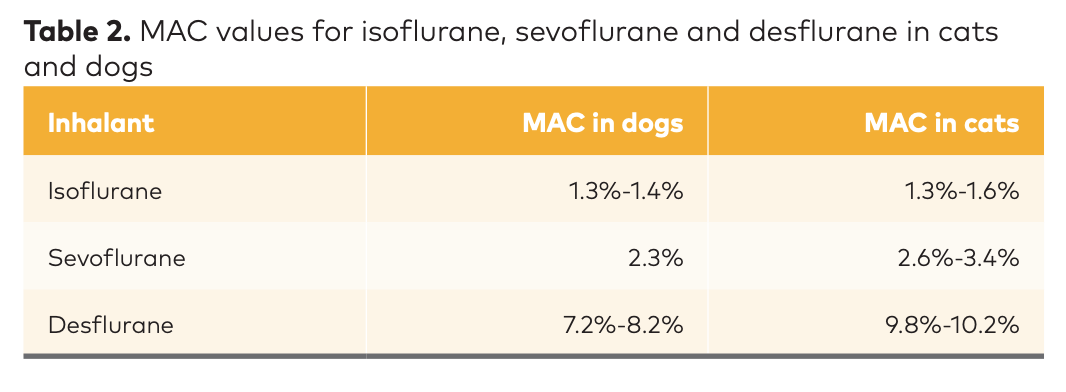
“Remember that this is the percent of inhalants the animal has to exhale,” notes Dr. Barletta. “That's the partial pressure we need to reach in the brain. In order to have 99% of animals not move, we need to multiply the MAC by 1.4 or 1.5.”
Isoflurane in dogs, for example, would be calculated like this: 1.4% x 1.5 = 2.1%. “That's pretty high for isoflurane, if you think that this isn't what you're dialing on your vaporizer,” he explains. “This is what's coming out of the animal. So you'd probably need to put the dial at 3.5% or 4% at the beginning of the anesthetic event. But because we're using so many other drugs, such as opioids, benzodiazepines and other sedatives and analgesics, this number goes way down.”
Most of the animals Dr. Barletta has anesthetized in the past four or five years have been on constant rate infusions of ketamine or fentanyl in order to decrease the amount of inhalant needed. “We're able to run isoflurane at around 1%, which is good because inhalants depress the patient's cardiovascular system,” he says. “The more you use, the more they do that. So I want to keep my inhalants low, but I still want to use the inhalants because I like the control.”
The beauty of the MAC is that inhalants do pretty much the same thing at equipotent MAC, says Dr. Barletta. “For example, at 1 MAC, the cardiovascular depression that you get from your isoflurane is the same as you get from sevoflurane and desflurane,” he explains. “So what isoflurane does in a dog at 1.3%, desflurane does at 7.2%. This makes comparison easy. If someone comes in to your clinic and says, ‘You've got to buy sevoflurane because it's so much more cardiovascular-friendly'-that's a lie.”
MAC interference
All anesthetic drugs that have anesthetic-sparing effects decrease MAC (i.e. increase potency), says Dr. Barletta. “That's why when we use some of these drugs (such as opioids) for premedication or during surgery, we can use less of the inhalant to achieve a surgical plane of anesthesia,” he explains. Other factors that decrease the MAC of an inhalant include age (geriatric patients), severe hypotension, hypoxemia, hypercarbia, hypothermia, hyponatremia, pregnancy and metabolic acidosis.
Knowledge for now … and later
Though Dr. Barletta's sessions focused on isoflurane, sevoflurane and desflurane, he says the same points of comparison and the rules that govern them will apply to new inhalants developed in the future as well-which will come in handy when their representatives come knocking.
Sarah Mouton Dowdy, a former associate content specialist for dvm360, is a freelance writer and editor in Kansas City, Missouri.
