How to handle feline aortic thromboembolism
Thromboembolism is a fairly common and potentially devastating complication of heart disease in cats.
Aortic thromboembolism (ATE) is a disease process whereby a thrombus is formed, typically in the left atrium. The thrombus then, either in part or as a whole, dislodges and travels (embolizes) through the aorta distally until it reaches an artery of small enough diameter that it can travel no farther. Heart disease is the most common cause of ATE in cats; two separate retrospective studies have identified cardiac disease in more than 90% and in 69% of cats with thromboembolism.1,2 Neoplasia and thyroid disease have also been associated with ATE.

Jupiter Images/Getty Images
This article discusses alterations in normal hemostasis that may predispose cats to thromboembolism; ATE's clinical presentation, diagnosis, and treatment; and possible prevention strategies.
INCIDENCE AND BACKGROUND
Thromboembolism is a fairly common and potentially devastating complication of heart disease in cats. ATE has been found in 12% to 28% of cats with hypertrophic cardiomyopathy (HCM) and 27% of cats with unclassified cardiomyopathy.2-5 Less commonly, ATE has been reported in cats with arrhythmogenic right ventricular cardiomyopathy and atrial fibrillation.6,7
Close to 90% of cats with systemic thromboembolism have a thrombus that lodges in the terminal aorta (aortic trifurcation) (Figures 1 & 2).8 Other potential sites for embolization include brachial, visceral, and cerebral arteries.8,9 Thrombi typically form in the left atrium or left auricle. A dilated left atrium is one of the biggest risk factors for ATE. In a study of cats with HCM, those with ATE had a significantly larger left atrial diameter than those that had congestive heart failure (CHF) alone or had subclinical disease.4 Arterial thromboembolism affects male cats more often than females, but cardiac disease more commonly affects males.2,9
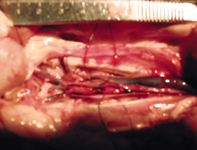
1. A photo taken at necropsy of a thrombus lodged at the aortic trifurcation. The cat's head is to the right.
Other diseases associated with thromboembolic disease in cats include neoplasia and thyroid disease. Pulmonary carcinoma causes embolic disease through both thromboembolism and embolization of tumor cells.2,10-12 Since cardiac disease is by far the most common cause of ATE in cats, we focus on ATE secondary to cardiac disease in this article.
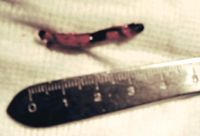
2. The excised thrombus from the cat in Figure 1.
PATHOPHYSIOLOGY OF ATE
Since the coagulation system plays a large role in ATE, a brief overview of normal hemostasis is needed to understand thrombus formation, treatment, and, maybe one day, prevention (see the Related Link "Normal hemostasis" below). Once a thrombus forms, it may stay static, grow, or embolize.
Thrombus formation
In general, three conditions favor thrombus formation: blood stasis, hypercoagulability, and endothelial damage. All three of these conditions, referred to as Virchow's triad, may be present in cats with myocardial disease.
Blood stasis. As the left atrium dilates, blood flow slows. Echocardiographically, this slow velocity appears as spontaneous echo contrast (SEC). SEC, or "smoke," is a phenomenon that arises from the slowing and sludging of blood, and this slowing results in the aggregation of red blood cells and other blood components. These clumps of cells and macromolecules often appear as flecks of echogenic material in the atrium and auricle.
Blood flow velocity in the left auricle can be estimated by using Doppler echocardiography.13 Decreased blood flow velocity in the left auricle has been demonstrated in cats with cardiac disease, and, in one study, 79% of cats with a decreased left auricle velocity had SEC.14
The presence of SEC may be a risk factor for ATE in cats. Several human studies have demonstrated patients with SEC to be at increased risk for stroke or other embolic events.15 However, no prospective studies in cats have specifically looked at SEC and risk for thromboembolism.
Hypercoagulability. Hypercoagulability has been documented in cats with cardiac disease. In a study that compared normal cats with asymptomatic cats with HCM, 45% of the cats with HCM had laboratory evidence that suggested hypercoagulability; however, they were not significantly different from the control group.16
Another study that evaluated only cats with HCM found that 56% and 50% of cats with ATE and SEC, respectively, had evidence of hypercoagulability.17 That study also provided evidence linking SEC with increased risk of thromboembolism.
Red blood cells and platelets in cats may also be more prone to aggregation than those in other species.18,19
Endothelial damage. Endothelial damage may also play a role in thrombus formation. Endothelial damage has been well-described in people with atrial fibrillation and predisposes them to thromboembolic events.20 Little information exists on endothelial damage and dysfunction in cats with cardiomyopathy. Endothelial function can be assessed by measuring circulating levels of biomarkers found in the endothelium such as von Willebrand factor, vascular cell adhesion molecules, and E-selectin.21 In one study, cats with ATE had significantly higher von Willebrand factor antigen concentrations than did cats with left atrial enlargement or left atrial enlargement with spontaneous echocardiographic contrast.17 However, that finding may have been a result of endothelial damage due to the thrombus itself.
Embolization
Three potential outcomes exist for a left atrial or auricular thrombus: It could remain static and cause no clinical signs, it could continue to grow and potentially interfere with the mitral valve orifice, or it could break off (partially or completely) and travel to distant parts of the body (embolism).8 Cats most commonly present with signs related to thromboembolism. The embolus usually follows the path of least resistance and lodges at the aortic trifurcation, occluding blood flow to both pelvic limbs. The thromboembolus may be small enough to only partially occlude one femoral artery, resulting in unilateral signs. Thromboemboli may also lodge in the renal arteries, resulting in renal failure.
The thrombus occludes the main artery and also impairs collateral circulation. In a study that compared cats that had their caudal aorta ligated vs. occluded with an experimentally induced thrombus, the ligated cats had only minimal neurologic deficits and 90% return of blood flow at 72 hours.22 The cats with the thrombus had deterioration of or no change in neurologic signs and had only 23% of preocclusion blood flow at 72 hours.22
Some factor related to the thrombus itself may inhibit collateral circulation. A likely theory is that the thromboembolus releases serotonin and thromboxane A2, causing vasoconstriction of the collateral vessels. Cats treated with serotonin and thromboxane antagonists before experimentally induced aortic thrombus formation had improved collateral circulation compared with the controls.23,24 Treatment with these agents after a thromboembolic event has no apparent benefit in terms of the initial thromboembolus but may prevent further thrombus formation.8
CONSEQUENCES OF THROMBOEMBOLISM
Tissue injury results not only from ischemia due to occlusion of blood flow but also from reperfusion as blood flow is restored.
Ischemia
Ischemia sets the stage for reactive oxygen species (ROS) formation and oxidative injury through a number of mechanisms.
Glycolysis predominates under anaerobic conditions, leading to lactate production, which results in acidosis. Depletion of ATP stores eventually leads to cell membrane pump failure, allowing potassium release from the cells and entry of sodium, calcium, and chloride. This electrolyte shift leads to cellular swelling and death. Hypoxic conditions also result in the production of various inflammatory mediators and adhesion molecules. Nitric oxide is inactivated and prostacyclin is inhibited, promoting vasoconstriction and platelet aggregation.25
Reperfusion injury
Reperfusion injury is worse than ischemic injury. As blood flow is re-established, the toxic byproducts and inflammatory mediators can circulate throughout the body. Potassium released from the cells enters the systemic circulation and can cause life-threatening hyperkalemia. As oxygen becomes available for ROS formation, ROS react with and damage virtually every molecule in the body including lipids, proteins, and DNA. The most reactive of these ROS trigger a self-perpetuating reaction of lipid peroxidation that results in increased membrane permeability and cell death.25
Nitric oxide also plays a role in ROS formation and cellular injury. Nitric oxide is produced by nitric oxide synthase (NOS). The inducible form of nitric oxide synthase (iNOS) is synthesized in response to hypoxia and inflammation. The iNOS can produce large amounts of nitric oxide, leading to severe vasodilation and hypotension.26 As NOS requires oxygen as a substrate, the damaging effects of iNOS may not occur until blood flow is re-established.25
Neutrophils are also important in the pathogenesis of reperfusion injury through ROS generation and proteolytic enzyme release.25 Excessive neutrophil recruitment also contributes to the "no reflow" phenomenon that is characterized by a decrease in capillary perfusion after blood flow is re-established as a result of endothelial cell swelling.27
CLINICAL SIGNS AND PHYSICAL EXAMINATION FINDINGS
Clinical signs of thromboembolism in cats develop acutely. Owners typically are unaware of any prior problems and report the cats were acting normally before the event.
Most owners first notice vocalization and then find the cats lying on the ground or having difficulty ambulating. Some owners report their cats may have been hiding before the incident. In many cases, the cat will be tachypneic or panting. Most cats have no previous history of heart disease. In one retrospective study, 90% of the cats had no prior diagnosis of heart disease.9 Many cats present with concurrent CHF.
Clinical signs depend on the site of the thromboembolus. Since the terminal aorta is the most common site of occlusion, most cats present for evaluation of pelvic limb paralysis or paresis. Both pelvic limbs are affected in most of these cats.9 On physical examination, the affected limb is usually cool to the touch compared with the other limbs, and the muscles are firm and painful. Pulses in the affected limb are typically absent but may be weak if the artery is only partially occluded.
Cardiac auscultation findings may support underlying heart disease such as a murmur, diastolic gallop sound, or arrhythmia. The heart rate is variable: cats may be tachycardic or bradycardic. Fine crackles or increased bronchovesicular sounds may be heard on auscultation of the lungs if pulmonary edema is present. These cats usually have normal hydration because of the acute nature of the condition unless presentation has been delayed.
DIAGNOSIS
The main differential diagnosis for aortic thromboembolism is spinal cord disease (acute spinal injury, intervertebral disk disease, spinal neoplasia, and fibrocartilaginous emboli). Definitive diagnosis involves documenting arterial occlusion and determining the underlying cause.
Blood flow evaluation
Various methods exist to evaluate blood flow (or lack thereof) to the limb. Palpation of pulses may not be a reliable indicator since pulses may be difficult to palpate in any situation of poor perfusion (such as hypovolemic shock). Blood flow in the artery can be assessed by applying a Doppler transducer over the dorsal pedal artery or palmar artery. Absence of a Doppler signal may indicate lack of blood flow. The accuracy may be affected by poor perfusion and hypotension due to other conditions.
Other means of assessing blood flow include measuring glucose or lactate concentrations in the affected limb by obtaining a blood sample from the medial saphenous vein and comparing it with the systemic values. The glucose should be lower and the lactate should be higher in the ischemic limb. Angiography provides the best information regarding blood flow, but this procedure is not practical and is rarely needed in most cases of thromboembolism.
Laboratory testing
A minimum database consisting of a complete blood count (CBC), serum chemistry profile, and urinalysis should be performed in all cases. The CBC and urinalysis findings are typically normal.
The serum chemistry profile may reveal nonspecific changes such as hyperglycemia and increased alanine transaminase and aspartate transaminase activities. The cats may be azotemic from decreased renal perfusion or occlusion of the renal artery by the thrombus. Hyperkalemia may be present on admission or may develop as treatment progresses related to concurrent renal infarction or reperfusion syndrome (released from ischemic skeletal muscle cells). The hyperkalemia can be severe and may require treatment. Other abnormalities include hypocalcemia, hyperphosphatemia, hypokalemia, and hyponatremia.2 Serum creatine kinase activity is also markedly increased.28
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) are usually normal on presentation.9 D-dimer concentrations (products of the breakdown of cross-linked fibrin) can be measured and have been found to be elevated in only up to 50% of cats with ATE.17 D-dimers are fairly nonspecific as they can be elevated with other causes of thrombosis (e.g. disseminated intravascular coagulation, neoplasia, hepatopathy, hemorrhage).
Thyroid testing may also be indicated since ATE has been reported in cats with hyperthyroidism.2
Radiography
A thoracic radiographic examination is beneficial even if pulmonary auscultation is normal. Cardiomegaly was documented in 88% of cats with ATE in one study.1 Cardiomegaly in a previously asymptomatic cat provides evidence of underlying cardiac disease. Pulmonary edema or pleural effusion may also be evident on thoracic radiographs, as well as support for pulmonary neoplasia.
Echocardiography
An echocardiographic examination is extremely valuable for determining whether cardiac disease is a cause of ATE. Echocardiography can also be used to look for evidence of additional thrombus formation. Occasionally, a thrombus may be seen in the left auricle or atrium. As previously discussed, SEC may be present, indicating increased risk for thrombus formation.
INITIAL TREATMENT CONSIDERATIONS
Often before specific treatment for ATE can begin, more life-threatening conditions need to be addressed.
Congestive heart failure
Critical initial treatment most often involves treating CHF. Standard treatment for CHF involves oxygen and furosemide. Most cats are tachypneic, but tachypnea does not always correlate with the presence of CHF. In one study, tachypnea or panting was present in 88% of cats without CHF.2 Pain and anxiety likely play a role in tachypnea in patients without CHF. Perform a thoracic radiographic examination to confirm pulmonary edema or pleural effusion before initiating treatment since diuresis caused by furosemide could impair perfusion.
Pain management
The ischemic neuromyopathy that results from ATE is extremely painful, so pain control is a priority. Most cats require an opioid to achieve adequate pain control. Hydromorphone (0.1 mg/kg intravenously every two to four hours),29 buprenorphine (0.02 mg/kg intravenously every six to eight hours),30 and fentanyl (2 to 3 μg/kg intravenous bolus followed by 2 to 3 μg/kg/hr continuous-rate infusion)31 are good choices. Butorphanol (0.2 to 0.4 mg/kg intravenously, intramuscularly, or subcutaneously)32 provides minimal analgesia but does have some sedative and anxiolytic effects.
Fluid therapy
Maintaining perfusion to the liver and kidneys will help counteract the toxic byproducts produced during ischemia. Fluid therapy may be needed in cats that do not have CHF, especially if the patient is dehydrated. Administer the fluids judiciously since most of these cats have cardiac disease and may be susceptible to the development of fluid overload. Nasoesophageal tube trickle feeding not only provides a source of nutrition but also is a source of free water that will maintain hydration without the risk of intravenous fluid overload.
Other supportive therapy
Positive inotropic agents or vasopressors may be needed in severely affected patients with depressed cardiac function or refractory hypotension, but they should be used with caution and with the full understanding of any impact on the underlying cardiac disease.
Treatment of severe hyperkalemia (serum potassium concentration > 8 mEq/L or symptomatic bradyarrhythmia) involves administering an intravenous bolus of 1 ml/kg 25% dextrose with or without an intravenous injection of 0.5 U/kg regular insulin. Refractory hyperkalemia may require the cautious administration of sodium bicarbonate at a dose of 0.5 to 2 mEq/kg given intravenously over 30 minutes.33
THROMBOEMBOLISM TREATMENT
While various specific treatment strategies have been used, none have proved more effective than supportive therapy alone, and some treatments have resulted in worse outcomes. The main therapeutic modalities specifically targeting the thrombus involve heparin, thrombolytic therapy, surgery, or combinations of the three modalities.
Heparin
Heparin is a polysulfated glycosaminoglycan anticoagulant that complexes with antithrombin to inhibit mainly factor IIa (thrombin) and factor Xa (factors IXa, XIa, and XIIa are also inhibited to a lesser degree).34 Heparin exists in two forms—unfractionated and fractionated or low-molecular-weight heparin (LMWH). Unfractionated heparin molecules are larger and have less predictable activity than LMWH molecules.34 LMWH molecules are small enough that they cannot bind thrombin and antithrombin at the same time, so only factor Xa is inhibited.34 This makes therapy with LMWH less likely to result in bleeding complications as factor II (thrombin) activity is not inhibited.
The current unfractionated heparin recommendation of 300 U/kg given subcutaneously every eight hours is based on a study in healthy cats that found that this dosing regimen achieved a heparin concentration similar to that which is considered therapeutic in people.35 No studies have been performed in cats to determine an effective plasma concentration.9
Unfractionated heparin therapy requires close monitoring because of a wide variation in bioavailability. Ideally, aPTT should be monitored with unfractionated heparin therapy, so a baseline coagulation profile should be a part of the minimum database. The goal is a prolongation of aPTT by 1.5 to 2.5 times baseline, which has been extrapolated from human medicine.2 Frequent monitoring of the aPTT has been advised, but standardized protocols have not been validated in cats.
Thrombolytic agents
Three thrombolytic drugs have been used in cats with thromboembolism—streptokinase, tissue plasminogen activator (TPA), and urokinase.
Streptokinase is produced from Streptococcus species bacteria.9 The drug results in fibrinolysis by binding to plasminogen, forming a complex that is capable of converting other plasminogen molecules to plasmin. Streptokinase causes nonspecific degradation of fibrinogen, prothrombin, and factors V, VII, and XII, which has the potential to cause excessive bleeding.36 Streptokinase has shown no therapeutic benefit and has resulted in severe side effects in cats. In a retrospective study of 46 cats with ATE, only 33% of treated cats were discharged from the hospital.37 Life-threatening hyperkalemia developed in 35% of cats, and 24% had bleeding complications. Since streptokinase can cause life-threatening complications and there is no evidence of improved survival time, streptokinase treatment is not recommended.
TPA and urokinase are endogenous thrombolytic agents that form a complex with fibrin to cleave plasminogen to plasmin. TPA is more selective in targeting thrombi and has less potential for bleeding than streptokinase because of its fibrin binding site.36 In a recent small prospective study of TPA in cats with ATE, only 27% of the cats survived to discharge.38 All cats in the study suffered adverse events such as azotemia (45%), neurologic signs (45%), arrhythmias (45%), hyperkalemia (36%), and acidosis (18%); sudden death occurred in one cat. The study was stopped early because of the number of severe side effects. Thus, treatment with TPA is not recommended.
A recent case report outlined successful thrombolysis using an infusion of urokinase directly at the thrombus site.39 Clinical trials are needed to determine whether this will become a worthwhile treatment.
Surgery
Historically, surgical removal of the clot has been attempted with poor outcomes. Another method of surgical treatment is catheter-directed thrombectomy. This procedure has been used successfully in people with thromboembolic disease. One study evaluated its use in six cats with ATE; half of the cats survived to discharge.40 Of the three that survived, two died within four months of the procedure. One cat died and one was euthanized, both while anesthetized. Other adverse events included hypotension, neurologic signs, and acidosis. Because of the severity of adverse events, this treatment is not recommended. Further research is needed to determine whether surgical procedures will be beneficial in cats with ATE.
PREVENTION
No strong recommendations exist for one particular prophylaxis strategy. Several drugs have been used to inhibit the coagulation cascade or platelets. These drugs have been used as single agents and in combination.
Warfarin
Warfarin was one of the first antithrombotic drugs used in human and veterinary medicine, and it is still widely used in people. Warfarin inhibits the vitamin K-dependent clotting factors II, VII, IX, and X as well as the anticoagulation factors protein C and protein S.41 Careful monitoring of coagulation parameters is needed to reduce the risk of bleeding complications. The PT is the monitoring tool of choice. In people, the goal is an international normalized ratio (INR) of 2.0 to 3.0.9 The INR is the standardization of PT to take into account variations among laboratories.
Pharmacodynamic studies in healthy cats suggest a dose of 0.06 to 0.09 mg/kg/day given orally.41 Patients must undergo anticoagulant therapy with heparin before starting warfarin therapy since inhibition of proteins C and S occurs first and may make patients initially hypercoagulable.34 No prospective randomized studies have been conducted on the efficacy of warfarin in cats with thromboembolic disease to justify its use since it has potential to produce lethal bleeding complications.
Heparin
Long-term heparin therapy is best accomplished with LMWH. Two products approved for use in people, Dalteparin (Fragmin—Eisai) and enoxaparin (Lovenox—Sanofi-Aventis), are used most commonly in cats to prevent ATE. Owners can be taught to give the injections at home just as with an insulin injection.
Current dosing guidelines are 100 U/kg twice daily subcutaneously for dalteparin and 1 mg/kg subcutaneously twice daily for enoxaparin.9 One retrospective study showed LMWH to be well-tolerated and resulted in minimal bleeding complications at a median dose of 99 U/kg.42 However, recent studies have shown that LMWH is rapidly absorbed and eliminated in healthy cats.43,44 These findings call into question the current dosing scheme. The dose and frequency may need to be increased. Therapy can be monitored by measuring anti-Xa activity after achieving a steady state 48 hours after initiating therapy. Although LMWH appears to be safe and well-tolerated in cats, no prospective randomized trials have been conducted to determine its effectiveness in preventing ATE.
Aspirin
Aspirin has been used for many years in virtually every thromboembolic disease process. Aspirin irreversibly inhibits platelets through inhibition of thromboxane A2 synthesis.45 Low-dose aspirin has been recommended in people to prevent potential hypercoagulability resulting from prostacyclin inhibition with higher (conventional dose) aspirin.45 Low-dose aspirin is inexpensive, fairly safe, and usually well-tolerated in cats. Doses range from 5 to 20.25 mg/cat every 72 hours.2 This low dose has been shown to produce similar survival time and recurrence rates with fewer side effects compared with higher doses.2
Thienopyridines
The thienopyridines are a new class of platelet inhibitors that act through antagonism of the adenosine diphosphate receptor.46 Antagonism of adenosine diphosphate inhibits serotonin production and may preserve collateral circulation and decrease the severity of signs in cats that develop repeat thromboembolic episodes. The two drugs in this class that have been used in cats are clopidogrel (Plavix—Bristol-Myers Squibb/Sanofi-Aventis) and ticlopidine (Ticlid—Roche Laboratories). Clopidogrel has a less severe side effect profile in people than ticlopidine has, consisting of mainly gastrointestinal signs.46
Both drugs have been studied in cats. A small study of ticlopidine in cats found it to be effective in impairing platelet function, but its use in cats is not recommended because of dose-dependent gastrointestinal signs.47 Clopidogrel has also been shown to be effective at decreasing platelet aggregation in cats at a dose of 18.75 mg/cat given orally every 24 hours.48,49 The ongoing FATCAT study is a double-blinded multi-site study evaluating the comparative efficacy of clopidogrel vs. conventional-dose (81 mg/cat every 72 hours) aspirin in preventing ATE recurrence, which should provide good information about the relative efficacy of these two pharmacotherapeutic approaches (http://www.vin.com/fatcat).
PROGNOSIS
The prognosis for cats with ATE remains guarded to poor. Most cats die or are euthanized as a result of the disease. Table 1 summarizes various treatment and survival times. Studies have shown survival to discharge rates of only 27% to 35% with various treatment strategies.1,2,37,38 Median survival times of cats surviving to discharge range from 117 to 345 days.1,2,4,37

Table 1: Previous Studies Outlining Initial Treatment of Cats with Aortic Thromboembolism, Percent Discharged from the Hospital, and Median Survival Time
Body temperature is one of the most important prognostic indicators; hypothermia at presentation has been consistently associated with poor prognosis. In one study, a model predicting survival showed the probability of survival was 50% with a rectal temperature of 98.9 F (37.2 C) and less than 25% at 96 F (35.6 C).2
The number of hindlimbs affected also impacts prognosis. Bilaterally affected cats have a worse prognosis than unilaterally affected cats.2 Cats with thromboemboli that affect the forelimbs have a better prognosis than those with thromboemboli that affect the hindlimbs.9
Concurrent CHF does not affect survival to discharge but reduces survival time.2 Cats are at high risk for future thromboembolic events as 25% to 47% experience future ATE episodes.1,2,37
CONCLUSION
ATE is a serious disease process in cats affecting close to 30% of cats with cardiac disease.4 Treatment involves pain control, prevention of continued thrombus formation, limiting the effects of reperfusion injury by maintaining tissue perfusion, and managing the underlying cause. Hypothermia at presentation is a negative prognostic indicator. Re-thrombosis is a major concern. Current recommendations for preventing future ATE episodes include aspirin therapy (at various doses), LMWH, and clopidogrel either alone or in combination. At this time, no preventive strategy has proved effective or superior to any other strategy. Long-term prognosis is guarded to poor as most cats will eventually die of complications of ATE or CHF.
Timothy Koors, DVM
H. Cecilia Marshall, DVM, DACVIM (cardiology)
Veterinary Specialty Services
1021 Howard George Drive
Manchester, MO 63021
REFERENCES
1. Laste NJ, Harpster NK. A retrospective study of 100 cases of feline distal aortic thromboembolism: 1977-1993. J Am Anim Hosp Assoc 1995;31(6):492-500.
2. Smith SA, Tobias AH, Jacob KA, et al. Arterial thromboembolism in cats: acute crisis in 127 cases (1992-2001) and long-term management with low-dose aspirin in 24 cases. J Vet Intern Med 2003;17(1):73-83.
3. Peterson EN, Moise NS, Brown CA, et al. Heterogeneity of hypertrophy in feline hypertrophic heart disease. J Vet Intern Med 1993;7(3):183-189.
4. Rush JE, Freeman LM, Fenollosa NK, et al. Population and survival characteristics of cats with hypertrophic cardiomyopathy: 260 cases (1990-1999). J Am Vet Med Assoc 2002;220(2):202-207.
5. Atkins CE, Gallo AM, Kurzman ID, et al. Risk factors, clinical signs, and survival in cats with a clinical diagnosis of idiopathic hypertrophic cardiomyopathy: 74 cases (1985-1989). J Am Vet Med Assoc 1992;201(4):613-618.
6. Fox PR, Maron BJ, Basso C, et al. Spontaneously occurring arrhythmogenic right ventricular cardiomyopathy in the domestic cat: a new animal model similar to the human disease. Circulation 2000;102(15):1863-1870.
7. Cote E, Harpster NK, Laste NJ, et al. Atrial fibrillation in cats: 50 cases (1979-2002). J Am Vet Med Assoc 2004;225(2):256-260.
8. Kittleson MD, Kienle RD. Thromboembolic disease. In: Small animal cardiovascular medicine. St. Louis, Mo: Mosby, 1998;540-547.
9. Smith SA, Tobias AH. Feline arterial thromboembolism: an update. Vet Clin North Am Small Anim Pract 2004;34(5):1245-1271.
10. Sykes JE. Ischemic neuromyopathy due to peripheral arterial embolization of an adenocarcinoma in a cat. J Feline Med Surg 2003;5(6):353-356.
11. Hogan DF, Dhaliwal RS, Sisson DD, et al. Paraneoplastic thrombocytosis-induced systemic thromboembolism in a cat. J Am Anim Hosp Assoc 1999;35(6):483-486.
12. Ibarrola P, German AJ, Stell AJ, et al. Appendicular arterial tumor embolization in two cats with pulmonary carcinoma. J Am Vet Med Assoc 2004;225(7):1065-1069, 1048-1069.
13. Schober KE, Maerz I. Doppler echocardiographic assessment of left atrial appendage flow velocities in normal cats. J Vet Cardiol 2005;7(1):15-25.
14. Schober KE, Maerz I. Assessment of left atrial appendage flow velocity and its relation to spontaneous echocardiographic contrast in 89 cats with myocardial disease. J Vet Intern Med 2006;20(1):120-130.
15. Black IW. Spontaneous echo contrast: where there's smoke there's fire. Echocardiography 2000;17(4):373-382.
16. Bedard C, Lanevschi-Pietersma A, Dunn M. Evaluation of coagulation markers in the plasma of healthy cats and cats with asymptomatic hypertrophic cardiomyopathy. Vet Clin Pathol 2007;36(2):167-172.
17. Stokol T, Brooks M, Rush JE, et al. Hypercoagulability in cats with cardiomyopathy. J Vet Intern Med 2008;22(3):546-552.
18. Ohta K, Gotoh F, Tomita M, et al. Animal species differences in erythrocyte aggregability. Am J Physiol 1992;262(4 Pt 2):H1009-H1012.
19. Helenski CA, Ross JN Jr. Platelet aggregation in feline cardiomyopathy. J Vet Intern Med 1987;1(1):24-28.
20. Watson T, Shantsila E, Lip GYH. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet 2009;373:155-166.
21. Krishnamoorthy S, Lim SH, Lip GY. Assessment of endothelial (dys)function in atrial fibrillation. Ann Med 2009;41(8):576-590.
22. Schaub RG, Meyers KM, Sande RD, et al. Inhibition of feline collateral vessel development following experimental thrombolic occlusion. Circ Res 1976;39(5):736-743.
23. Schaub RG, Meyers KM, Sande RD. Serotonin as a factor in depression of collateral blood flow following experimental arterial thrombosis. J Lab Clin Med 1977;90(4):645-653.
24. Schaub RG, Gates KA, Roberts RE. Effect of aspirin on collateral blood flow after experimental thrombosis of the feline aorta. Am J Vet Res 1982;43(9):1647-1650.
25. McMicheal M, Moore RM. Ischemia-reperfusion injury pathophysiology, part I. J Vet Emerg Crit Care 2004;14(4):231-241.
26. Cauwels A. Nitric oxide in shock. Kidney Int 2007;72(5):557-565.
27. Seal JB, Gewertz BL. Vascular dysfunction in ischemia-reperfusion injury. Ann Vasc Surg 2005;19(4):572-584.
28. Aroch I, Keidar I, Himelstein A, et al. Diagnostic and prognostic value of serum creatine-kinase activity in ill cats: a retrospective study of 601 cases. J Feline Med Surg 2010;12(6):466-475.
29. Wegner K, Robertson SA, Kollias-Baker C, et al. Pharmacokinetic and pharmacodynamic evaluation of intravenous hydromorphone in cats. J Vet Pharmacol Ther 2004;27(5):329-336.
30. Robertson SA, Lascelles BD, Taylor PM, et al. PK-PD modeling of buprenorphine in cats: intravenous and oral transmucosal administration. J Vet Pharmacol Ther 2005;28(5):453-460.
31. Lamont LA. Feline perioperative pain management. Vet Clin North Am Small Anim Pract 2002;32(4):747-763, v.
32. Robertson SA, Lascelles BDX, Taylor P. Effect of 0.1, 0.2, 0.4 and 0.8 mg/kg of IV butorphanol on thermal antinociception in cats. Vet Anaesth Analg 2003;30:108.
33. DiBartola S. Disorders of potassium. In: Fluid, electrolyte, and acid-base disturbances. St. Louis, Mo: Elsevier Health; 2006.
34. Lunsford KV, Mackin AJ. Thromboembolic therapies in dogs and cats: an evidence-based approach. Vet Clin North Am Small Anim Pract 2007;37(3):579-609.
35. Kellerman DL, Lewis DC, Meyers NC, et al. Determination of a therapeutic heparin dosage in the cat. (abstr). J Vet Intern Med 1996;10:231.
36. Thompson MF, Scott-Moncrieff JC, Hogan DF. Thrombolytic therapy in dogs and cats. J Vet Emerg Crit Care 2001;11(2):111-121.
37. Moore KE, Morris N, Dhupa N, et al. Retrospective study of streptokinase administration in 46 cats with arterial thromboembolism. J Vet Emerg Crit Care 2000;10(4):245-257.
38. Welch KM, Rozanski EA, Freeman LM, et al. Prospective evaluation of tissue plasminogen activator in 11 cats with arterial thromboembolism. J Feline Med Surg 2010;12(2):122-128.
39. Koyama H, Matsumoto H, Fukushima RU, et al. Local inter-arterial administration of urokinase in the treatment of a feline distal aortic thromboembolism. J Vet Med Sci 2010;72(9):1209-1211.
40. Reimer SB, Kittleson MD, Kyles AE. Use of rheolytic thrombectomy in the treatment of feline distal aortic thromboembolism. J Vet Intern Med 2006;20(2):290-296.
41. Smith SA, Kraft SL, Lewis DC, et al. Pharmacodynamics of warfarin in cats. J Vet Pharmacol Ther 2000;23(6):339-344.
42. Smith CE, Rozanski EA, Freeman LM, et al. Use of low molecular weight heparin in cats: 57 cases (1999-2003). J Am Vet Med Assoc 2004;225(8):1237-1241.
43. Alwood AJ, Downend AB, Brooks MB, et al. Anticoagulant effects of low-molecular-weight heparins in healthy cats. J Vet Intern Med 2007;21(3):378-387.
44. Vargo CL, Taylor SM, Carr A, et al. The effect of a low molecular weight heparin on coagulation parameters in healthy cats. Can J Vet Res 2009;73(2):132-136.
45. Clarke RJ, Mayo G, Price P, et al. Suppression of thromboxane A2 but not of systemic prostacyclin by controlled-release aspirin. N Engl J Med 1991;325(16):1137-1141.
46. Quinn MJ, Fitzgerald DJ. Ticlopidine and clopidogrel. Circulation 1999;100(15):1667-1672.
47. Hogan DF, Andrews DA, Talbott KK, et al. Evaluation of antiplatelet effects of ticlopidine in cats. Am J Vet Res 2004;65(3):327-332.
48. Hogan DF, Andrews DA, Green HW, et al. Antiplatelet effects and pharmacodynamics of clopidogrel in cats. J Am Vet Med Assoc 2004;225(9):1406-1411.
49. Hamel-Jolette A, Dunn M, Bedard C. Plateletworks: a screening assay for clopidogrel therapy monitoring in healthy cats. Can J Vet Res 2009;73(1):73-76.