IMHA: Diagnosing and treating a complex disease
A careful diagnostic process will help you determine whether your veterinary patients will require long-term immunosuppressive therapy.
Autoimmune hemolytic anemia, or immune-mediated hemolytic anemia (IMHA), is a complex disease in which hemolysis occurs because of antierythrocyte antibody production. This article explores the pathophysiology of primary and secondary IMHA and diagnostic and treatment options, as well as prognosis in dogs and cats. Our review of the recent literature regarding IMHA in veterinary patients reveals a focus on individual cases and a lack of controlled clinical studies, which makes a detailed review of IMHA triggers and treatment options difficult.
Kip Carter
HEMOLYTIC ANEMIA VS. IMHA
Many causes of anemia exist in dogs and cats, so a clear distinction should be drawn between hemolytic anemia and IMHA.
Hemolytic anemia
Hemolytic anemias are conditions in which red blood cells (RBCs) are destroyed at an accelerated rate and a normal regenerative response is seen in the bone marrow. In these non-immune-mediated conditions, RBCs can be destroyed as a result of inherited membrane and enzyme defects, increased fragility from oxidative damage, or metabolic causes such as hypophosphatemia or water intoxication.1 Traditional immune-mediated mechanisms (immunoglobulins, complement) do not mediate lysis in hemolytic anemia. Instead, destruction occurs because of factors such as increased osmotic fragility, decreased RBC function in an alkalemic environment, or increased clearance from oxidative damage.1 Unlike the treatment of immune-mediated anemia, immunosuppression is generally not used to treat hemolytic anemias. Thus, it is imperative to investigate whether an anemia has an underlying cause before assuming it is immune-mediated.
Below we discuss some of the common causes of hemolytic anemia. A more exhaustive list can be found in Table 1.

Table 1: Selected Causes of Canine and Feline Non-immune-mediated Hemolytic Anemia
Zinc and copper toxicosis. One of the most common causes of hemolytic anemia in dogs is zinc toxicosis from the ingestion of zinc-containing objects. High zinc concentrations can be found in pennies minted since 1983, board game pieces, zippers, zinc oxide ointment, and various other sources. Zinc toxicosis can cause a severe intravascular hemolysis that is associated with small amounts of Heinz body and spherocyte formation. Hemolysis from zinc toxicosis can be easily mistaken for IMHA if a survey abdominal radiographic examination is not performed. Treatment consists of removing the foreign object and providing supportive care. Copper toxicosis can also result in a marked intravascular hemolysis and methemoglobinemia.1
Heinz body anemia. Heinz bodies are dark-staining refractile material that indicate irreversibly denatured and precipitated hemoglobin in RBCs and can occur from oxidative damage in animals that have ingested onions or received drugs such as methylene blue, dl-methionine, or vitamin K3.1 In some cases of hemolytic anemia, eccentrocytes—cells in which the damaged hemoglobin is clustered together and shifted to one side of the RBC membrane, leaving a clear crescent-shaped region—are also present. Eccentrocytes and cells containing Heinz bodies have less deformability and more rigidity, making them more likely to be lysed or removed from the circulation by the spleen.
Feline RBCs are especially sensitive to oxidative damage because of a high number of sulfhydryl groups in their hemogloblin. In addition, feline spleens, because of their nonsinusal conformation, are less effective at removing Heinz bodies than are canine sinusal spleens. Thus, Heinz body anemia is more commonly seen in cats and can be present during toxicosis as well as in a variety of other diseases such as hyperthyroidism, diabetes mellitus, and lymphoma.1
Hypophosphatemia. Hypophosphatemia can also cause hemolytic anemia in patients being treated for diabetes mellitus, hepatic lipidosis, starvation, and other conditions.1 In these cases, it is thought that an abrupt drop in plasma phosphate concentrations can cause a concurrent depletion of RBC adenosine triphosphate, diphosphoglycerate, and reduced glutathione. These depletions lead to less deformability, more osmotic fragility, and more susceptibility to oxidative injury in erythrocytes. A rapid drop in packed cell volume and a mild Heinz body anemia can be seen in animals with hypophosphatemia. Treatment consists of phosphate supplementation (intravenous in cases of severe depletion, oral in mild cases).1
Primary and secondary IMHA
IMHA has been described in people, dogs, cats, and a wide range of other species.2 In dogs, it is estimated that 60% to 75% of cases are considered primary (idiopathic), meaning that no underlying cause can be found.3 It is thought that as more intense clinical investigations are conducted in these patients, underlying diseases and triggers will be discovered.3 Any breed can be affected with primary IMHA, but certain breeds—such as cocker spaniels, poodles, Irish setters, and Old English sheepdogs—seem to be overrepresented (Table 2).1 In both people and dogs, there appears to be a linkage between specific human leukocyte antigen (HLA) and dog leukocyte antigen (DLA) haplotypes and autoimmune disease, although the genes involved may differ for each disease. A recent study in dogs investigated whether a genetic mutation in the DLA of certain breeds predisposes them to IMHA. The results suggest that more than one gene may be involved in these susceptible breeds.4

Table 2: Selected Causes of Canine and Feline IMHA
In secondary IMHA, RBC destruction occurs as a consequence of the immune system reacting to some condition or being activated by an agent. The end result is that the RBCs are destroyed as innocent bystanders. The process is probably the result of several factors, including an animal's susceptibility to disease and its propensity to form an autoimmune response. Usually, the process results from a combination of environmental factors and a genetic predisposition. The cellular mechanisms by which an agent can cause autoimmunity can include infection of immune cells, activation of lymphocytes from exposure to cytokines, cross-reactivity between microorganisms and host tissues, and the production of drug-specific antibodies with the formation of immune complexes.2 The loss of self-tolerance, resulting in autoimmunity, is also a mechanism by which hemolytic anemia can occur.
A recent report linked the class III antiarrhythmic agent amiodarone with a positive Coombs test result in two dogs. One of these patients developed hemolytic anemia, the other developed thrombocytopenia.5 Another report discussed intravascular hemolysis associated with a patent ductus arteriosus coil embolization in a dog.6 Examples of possible underlying conditions causing secondary IMHA are listed in Table 2 and include infections, drugs, and neoplasia, as well as other immune-related disorders. Secondary IMHA is the common type of immune-mediated hemolysis in cats.1
In certain infectious diseases, such as mycoplasmosis and babesiosis, hemolysis caused by the organism is exacerbated by the body's own immune response. If you suspect an infectious disease, administer antibiotics pending the results of antibody titers or polymerase chain reaction (PCR) testing. Additionally, to reduce the body's response to the infectious agent, immunosuppressive medications often need to be administered. Once an underlying infectious agent is identified, the immunosuppressive medications can be used at lower doses and for an abridged treatment course.
PATHOPHYSIOLOGY
IMHA is a type II hypersensitivity reaction in which RBCs are destroyed by the body's own immune system. The process typically involves a breakdown of immune self-tolerance and the production of anti-erythrocyte antibodies, IgG, or IgM. These antibodies are often recognizing and reacting to RBC membrane glycoproteins.7 Erythrocyte destruction is initiated when the surface becomes coated with complement and either IgM or IgG.8
The most common form of IMHA is IgG-mediated. The IgG-coated RBCs are destroyed by macrophages located in the liver or spleen (extravascular). Macrophages either consume the entire erythrocyte or remove a portion of the membrane, leaving smaller RBCs with no central pallor (spherocytes). These rigid spherocytes are then trapped in the spleen and destroyed. IgM-coated RBCs will also activate complement more efficiently than IgG, with destruction generally occurring within vessels (intravascular).1
SIGNALMENT, HISTORY, AND CLINICAL SIGNS IN DOGS
The age of onset of IMHA in a patient varies; however, the disease frequently occurs in young to middle-aged dogs. Females may be predisposed to developing IMHA.

Rules of thumb for diagnosing and treating IMHA in dogs and cats
Patients can have an acute or a chronic history of malaise. Vomiting or diarrhea may occur before the classic signs of anemia, which are lethargy, inappetence, polyuria, polydipsia, pallor, tachypnea, and changes in urine color. Clinical signs can include weakness, pale or icteric mucous membranes, bounding pulses, tachypnea, tachycardia, hepatosplenomegaly, or a heart murmur. A patient's clinical signs may be related to an underlying disease process rather than the anemia itself.1
To investigate potential underlying causes of IMHA, obtain a thorough and specific history. Ask questions regarding a wide range of environmental and historical circumstances to determine the following:
1. Propensity to ingest foreign objects
2. Diet and treat history (e.g. recent ingestion of onions or garlic)
3. Recent history of vaccine or drug administration
4. Travel history
5. History of transfusions or recent dog fights
6. Recent tick exposure or bee stings
7. Heartworm preventive status
8. Reproductive status
9. Signs related to other underlying disease, such as hematemesis (zinc-containing foreign body), vaginal discharge (pyometra), or weight loss and malaise (neoplasia).
DIAGNOSTIC TESTS IN DOGS
Baseline tests recommended for diagnosing IMHA and investigating causes of secondary IMHA include a complete blood count, a reticulocyte count, a serum chemistry profile, a blood smear, a slide agglutination test, a direct Coombs test, and abdominal and thoracic radiography.
Complete blood count
Complete blood count abnormalities usually include a regenerative anemia, although as many as 33% to 50% of dogs with IMHA can have nonregenerative anemia because of an immune attack at the level of the bone marrow or early disease.9,10 A mild to marked increase in the white blood cell count can be seen, occasionally with a left shift and toxic neutrophils. This leukocytosis can be a result of many factors, including glucocorticoid-induced leukocytosis, anemic hypoxia, thromboembolic disease, and tissue necrosis.11 A patient presenting with concurrent severe thrombocytopenia (< 50,000 platelets/µl) can have clinical signs such as petechiation, ecchymosis, epistaxis, or melena. Low platelet counts have been associated with a greater risk of thromboembolism and a higher mortality rate in dogs.12
Serum chemistry profile
Serum chemistry profile abnormalities may reflect organ damage from hypoxia while also indicating an underlying disease process. Elevated liver enzyme activities (alkaline phosphatase, alanine transaminase, and aspartate transaminase) and serum bilirubin concentrations are common.13 Even before glucocorticoid administration, many patients can have elevated liver enzyme activities from hepatic hypoxia, inflammation, or necrosis.13 Bilirubin concentrations can also be normal if the hemolysis has been chronic and the liver has had time to metabolize the bilirubin.1 Some studies have found a correlation between elevated bilirubin concentrations and increased mortality, while others have not.12-14 Other serum chemistry abnormalities may reflect underlying conditions.
A recent study concluded that an increased blood urea nitrogen concentration and band neutrophils, a decrease in platelets, and petechiation at the time of diagnosis were all negative prognostic indicators in dogs presenting with primary IMHA.3 Anecdotally, the presence of intravascular hemolysis also appears to impart a poorer prognosis in these patients.
Macroagglutination and blood smear evaluations
Preparing two blood slides is imperative in diagnosing IMHA and should be performed at the outset in every suspected case. On the first slide, mix a drop of blood with a drop of saline solution, gently agitate the mixture, and then visually inspect it for macroagglutination. On the second slide, you can evaluate a blood smear in-house and also send it to a clinical pathologist for evaluation of spherocytes (Figure 1), Heinz bodies, blood parasites, and microagglutination (Figure 2). Spherocytes and erythrocyte macroagglutination and microagglutination are commonly found on blood smears in patients with IMHA. As many as 89% of dogs with IMHA have spherocytes; however, dogs with secondary hemolysis from hypophosphatemia, zinc intoxication, or splenic disease can also have them in small numbers.1,14
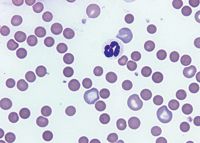
1. A peripheral blood smear from an anemic dog reveals that the majority of the RBCs are small, dark, and lack central pallor, which indicates they are spherocytes. Several large polychromatophilic cells are also present, which indicate a regenerative response to the anemia (Wright's-Giemsa stain; 1000X)
Direct Coombs test
The direct Coombs test, also called the direct antiglobulin test, can be used when IMHA is suspected but autoagglutination is not seen. This test is run on an EDTA blood sample and will identify antibodies or complement on a patient's RBC surface. A positive result on a direct Coombs test does not distinguish between primary and secondary causes of IMHA.1 False positive results are seen in both dogs and cats and can be a result of concurrent diseases such as neoplasia, infections, inflammatory conditions, or recent drug administration.15 False negative results are also common and can occur in up to 42% of all dogs with IMHA.16 Thus, this test can be prone to errors and is not sensitive enough to detect low levels of clinically relevant antibodies.9 Flow cytometry also detects antierythrocyte antibodies and is more sensitive for detecting IgG than is the Coombs test, but it is not yet widely available to general practitioners.15,17
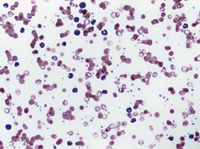
2. A peripheral blood smear from an anemic dog reveals that the RBCs are prominently clumped together, which indicates agglutination. A saline dispersion test should be performed for confirmation. Additional findings in this smear include moderate polychromasia and rare nucleated RBCs, which indicate a regenerative response, small round cells lacking central pallor suggestive of spherocytes, and a neutrophilic leukocytosis, which indicates inflammation (Wright's-Giemsa stain; 500X).
Imaging
Obtain abdominal radiographs to investigate triggers of hemolytic anemia, such as ingestion of zinc-containing foreign objects or abdominal neoplasia. If abdominal radiographs reveal suspicious findings, an abdominal ultrasonographic examination is warranted. In middle-aged to older dogs, it is recommended that routine thoracic radiographs also be obtained to investigate whether primary or metastatic neoplasia may be a cause of IMHA.
Other diagnostic tests
A thorough search for underlying infection is warranted in cases of IMHA. Chronic infections, such as pyometra, abscesses, urinary tract infections, and discospondylitis, have all been associated with triggering IMHA.1 Transmissible causes of IMHA, such as infection with the hemoprotozoan Babesia gibsoni, are increasingly frequent in the United States.18 A recent study described a breed predisposition to B. gibsoni infection in American pit bull terriers.18 Studies have also described transmission associated with blood transfusions and dog bites.19-21 Other infectious organisms, such as Ehrlichia and Dirofilaria species, have also been associated with anemia in dogs.22,23 Mycoplasmosis does not cause a clinically relevant anemia in dogs that have not undergone a splenectomy, but it can cause a mild to severe anemia in cats.1
A coagulation panel is justified to investigate blood loss as a cause of anemia or as an indicator of disseminated intravascular coagulation (DIC).24 Elevations in coagulation times, however, have not been shown to predict thromboembolism or mortality in patients with IMHA. In one study in dogs with IMHA, no statistical difference was noted in survival times in patients with or without baseline coagulation abnormalities.25
An antinuclear antibody (ANA) test is occasionally performed in patients with IMHA to investigate systemic lupus erythematosus; but to truly have this condition, patients must have high ANA titers and two or more manifestations of autoimmunity (e.g. dermatomyositis, vasculitis, polyarthropathy) in addition to IMHA.26 False positive results can occur with certain underlying infections and neoplastic diseases.
A bone marrow aspirate or core biopsy may be helpful in an ongoing nonregenerative anemia to search for underlying diseases such as RBC aplasia or hypoplasia, immune destruction of RBC precursors, or neoplastic infiltration.24
TREATMENT IN DOGS
The decision to hospitalize a patient with IMHA depends largely on the severity of clinical signs. If the disease is caught early and the patient is stable, close outpatient management can be a reasonable option. More often, patients presenting with IMHA are already quite sick and need hospitalization for monitoring, supportive care, and treatment.
The treatment of IMHA is extremely case-specific but generally involves four principles: 1) preventing hemolysis with immunosuppressive therapy, 2) treating tissue hypoxia, 3) deterring the formation of thromboemboli, and 4) providing supportive care.
Immunosuppressive therapy
Numerous drugs have been described to treat IMHA (Table 3). The first line of treatment in IMHA is immunosuppressive corticosteroids. The addition of secondary and tertiary immunosuppressants may be indicated when patients present with severe IMHA (very low hematocrits, severe autoagglutination, intravascular hemolysis, or thrombocytopenia), when glucocorticoids do not adequately control the hemolysis, or when the side effects of glucocorticoids become unacceptable. The use of multiple cytotoxic drugs in combination requires careful monitoring, however, as there is a risk of severe immunosuppression and danger of infection.27
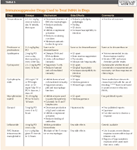
Table 3: Immunosuppressive Drugs Used to Treat IMHA in Dogs
Corticosteroids. Dexamethasone (0.1 to 0.3 mg/kg intravenously once or twice daily) can be given initially if oral medications cannot be tolerated. Prednisone or prednisolone (2 mg/kg orally once or twice daily) is the oral drug of choice. Glucocorticoids are thought to prevent hemolysis by decreasing the clearance of antibody-coated RBCs by macrophages, reducing the amount of antibody binding and complement activation on RBCs, and, in the long-term, minimizing autoantibody production.1 Side effects include polyuria, polydipsia, polyphagia, increased panting, gastrointestinal ulceration, and increased susceptibility to infection.
Azathioprine. In cases of severe IMHA (low RBC counts, autoagglutination, or intravascular hemolysis), the second drug usually added in dogs is azathioprine (2 mg/kg orally once daily or every other day). Studies have shown increased survival times in dogs treated with glucocorticoids and azathioprine.13,28 Drawbacks to this medication are that it is only available in oral form and it can take weeks to take effect.
Azathioprine is a purine analogue antimetabolite that disrupts DNA and RNA synthesis and is effective in limiting cell-mediated immunity (T-lymphocyte function).24 Side effects can include gastrointestinal upset, bone marrow suppression, pancreatitis, and hepatotoxicity. Regular physical examinations and complete blood counts and serum chemistry profiles (weekly at first, then monthly) will help to monitor for the more serious complications of this medication. Azathioprine can cause severe bone marrow and hepatic toxicosis in cats and is not recommended.24
Cyclosporine. A third drug gaining acceptance in treating IMHA is cyclosporine (2.5 to 5 mg/kg orally twice daily). An intravenous form is available, but no clinical trials with this form have been published in the veterinary literature. The one retrospective study that investigated cyclosporine (which was given at a slightly higher than recommended dosage) showed no benefit compared with other drug protocols.29
Cyclosporine is a potent T-cell suppressor that blocks production of immune activating factors in both T-helper cells and T-cytotoxic cells, as well as blocks the expression of IL-2 and gamma-interferon. The outcome is a reduction of cell-mediated immunity and antibody production. Side effects include gastrointestinal upset, gingival hyperplasia (can regress with dose tapering), increased susceptibility to infection, and lethargy. Although it is recommended that cyclosporine concentrations are measured every two to four weeks (maintaining trough concentrations, depending on the method used, between 100 and 300 ng/ml), it is now questionable whether blood concentrations of this drug need to be measured if clinical disease is well-controlled.17
Cyclophosphamide. In addition to the above therapeutic options, a handful of other medications have been used with varying degrees of success. Cyclophosphamide, an alkylating agent that suppresses the immune system, can be given as a single intravenous or oral dose initially in the course of treatment (200 mg/m2 ) or as a four days on/three days off (50 mg/m2 /day) protocol. Cyclophosphamide harms B and T cells by cross-linking DNA, inhibiting humoral and cell-mediated immunity, and suppressing neutrophil and macrophage function. Side effects include gastrointestinal upset, myelosuppression, and hemorrhagic cystitis. Because of the severity of some of these signs, cyclophosphamide is not widely used in the long-term treatment of IMHA. Instead, it is commonly used in early treatment of severe refractory IMHA.
Cyclophosphamide has fallen out of favor since a randomized, controlled, prospective clinical trial published in 2003 showed no improved recovery or survival times in patients treated with cyclophosphamide and prednisone vs. prednisone alone.30 Many other studies have also documented decreased survival in patients treated with cyclophosphamide.13,29,30
Mycophenolate mofetil. Mycophenolate mofetil (10 to 20 mg/kg orally or intravenously twice daily), similar to azathioprine, targets B and T lymphocytes by inhibiting an enzyme necessary for de novo purine biosynthesis.15 This drug was developed to help prevent allograft rejection in people and has been used in dogs to treat myasthenia gravis and glomerulonephritis. At therapeutic doses, mycophenolate is minimally myelosuppressive, but gastrointestinal side effects can be substantial in dogs (gastrointestinal hemorrhage, anorexia, and diarrhea). To minimize these side effects, the dose can be lowered to well-tolerated levels when used in conjunction with other myelosuppressive drugs.31 Few reports have been published on the use of this drug for IMHA.32
Danazol. Danazol (5 mg/kg orally two to three times daily), a synthetic androgen, has been used to treat
immune-mediated disease in people and is sometimes recommended in addition to standard therapies to treat IMHA in dogs. Danazol likely exerts its immunomodulatory effects by decreasing the production of IgG and cytokines, inhibiting complement activation, and reducing the binding of antibody and complement to erythrocytes.24 Danazol is not widely used for three main reasons: it is expensive, it can take weeks to observe a clinical response, and it is potentially hepatotoxic.1 While one study did not show improved outcome with this drug, the small number of reports in the veterinary literature makes the use of this drug difficult to assess.33
Leflunomide. Leflunomide (4 mg/kg orally once daily), an inhibitor of pyrimidine biosynthesis, has been used to treat rheumatoid arthritis in people and granulomatous meningoencephalomyelitis, neoplasia, and graft rejection in dogs. Limited but favorable reports exist on the use of this drug for treating IMHA. Side effects in dogs appear to be minimal but can include vomiting, lymphopenia, and anemia. The recent introduction of a generic form may make it less costly for routine treatment. The dose should be adjusted to maintain a serum trough concentration of 20 µg/ml.31
IVIG. Intravenous human immunoglobulin (IVIG) (0.5 to 2 g/kg intravenously daily, given over six to 12 hours) has been infrequently used to treat a variety of immune-mediated diseases in dogs, including IMHA. The mechanism of action of IVIG is thought to be a blockade of the Fc receptors on macrophages, thereby reducing phagocytosis of antibody-coated RBCs, interfering with complement, and suppressing antibody production. In addition, IVIG inhibits erythrocyte phagocytosis by binding to canine monocytes and lymphocytes and possibly by an anti-idiotypic down-regulation of antibody production.
IVIG can be given to patients with IMHA as a single infusion or on two or three consecutive days.34 Although IVIG appears to impart short-term benefits (reflected by a rising packed cell volume and reticulocytosis) within days of infusion, long-term benefits were not seen. No complications have been seen with a single IVIG infusion in dogs. Unfortunately, IVIG is also costly and difficult to obtain.30
Liposomal-encapsulated clodronate. Liposomal-encapsulated clodronate (dichloromethylene diphosphonate) is the focus of two studies, one of which is ongoing at Colorado State University.35,36 Clodronate is a bisphosphonate that, when incorporated into liposomes, is rapidly phagocytized by macrophages leading to apoptosis. Intravenous liposomal-encapsulated clodronate has been shown to significantly reduce the number of canine splenic macrophages and dendritic cells in vitro, thereby obstructing the clearance of antibody-coated RBCs. This reduction, in effect, slows the clearance of opsonized erythrocytes and allows time for the other immunosuppressive drugs to work. The initial study, involving intravenous infusion of liposomal-encapsulated clodronate in healthy dogs and in seven dogs with IMHA, found that the drug is rapid-acting and well-tolerated.35 In that study, results were favorable, and dogs treated with liposomal-encapsulated clodronate in conjunction with prednisone, azathioprine, and heparin demonstrated an improved survival.
Splenectomy
Splenectomy is considered one of the last-choice treatments in canine IMHA. The benefits arise from removing one source of B cells and splenic macrophages, the primary culprits in the removal of antibody-coated erythrocytes.24 It is undetermined how effective this procedure is in routine IMHA cases, since only one recent clinical study has been done to assess it as a forerunner of treatment.37 Although this study did show increased survival with splenectomy (58% survival vs. 37.5% in the control group), the sample size was small.
Consider splenectomy only in patients that have not responded to immunosuppressive medications, require high-dose and long-term medications to maintain a remission, or are experiencing severe side effects from medications. As there is a risk of developing marked infection after splenectomy, it is not recommended for patients taking multiple immunosuppressive medications.1
Treating tissue hypoxia
Patients with IMHA often need oxygen-carrying fluids to support them through the first few days of care until immunosuppression begins to control the disease process. Oxygen alone is rarely beneficial in severe anemia, unless a patient's clinical signs are complicated by thromboembolism. The criteria for transfusion are not rigid but may include the presence of severe tachypnea, dyspnea, tachycardia, cold extremities, weakness, mental depression, or a hematocrit under 15%. If a patient appears comfortable when resting in a cage but becomes agitated and clinically unstable during necessary procedures (radiography or ultrasonography), give a transfusion before proceeding.
The choice of administering packed RBCs, whole blood, or synthetic hemoglobin can be controversial and is often institution-dependent. Ideally, only the necessary component should be given. As transfusion reactions can occur in patients that have not had transfusions previously, crossmatching should be done before transfusion in all non-autoagglutinating patients. Also perform cross-matching in any dog that has received a prior blood transfusion. Since autoagglutination may interfere with accurate blood typing and crossmatching, packed RBCs should only be administered from universal donors (DEA 1-7 negative blood; DEA 4 can be positive) in dogs with IMHA.15 Administering purified polymerized bovine hemoglobin solution (Oxyglobin—Biopure) can be beneficial in cases in which crossmatching is not possible or compatible blood is not available. Although one study noted that Oxyglobin administration was associated with a poorer prognosis, a more recent study contradicted those findings.12,29
Thromboembolism and anticoagulants
DIC and thromboembolism are common causes of morbidity and mortality in patients with IMHA.1 The pathogenesis is largely unknown, but potential contributors include endogenous conditions such as blood flow stasis and hypercoaguability as well as exogenous factors such as repeated venipuncture, intravenous catheters, and glucocorticoid administration. Whether anticoagulant therapy is used at the time of diagnosis or added when thromboembolism is suspected is largely institution-dependent. Anticoagulants such as low-molecular-weight heparin, unfractionated heparin, warfarin, low-dose aspirin, and fresh-frozen plasma have all been used to prevent or treat this condition, although few studies have shown prolonged survival with their use.15
In one study, the use of fresh-frozen plasma (10 ml/kg) with unfractionated heparin (100 U/kg subcutaneously every six hours) failed to prevent thromboembolic complications in IMHA patients.38 Unfractionated heparin alone is generally not associated with an increased risk of bleeding, but neither is it associated with an improved outcome. Two studies failed to document increased survival with the use of unfractionated heparin.13,14 Another study demonstrated that relatively high doses of unfractionated heparin (300 to 375 U/kg subcutaneously every six hours) were needed to obtain target concentrations for anticoagulation, and even those concentrations may be inadequate in preventing thrombosis.39 If used, unfractionated heparin should be adjusted to prolong the activated partial thromboplastin time to 25% to 50% over baseline or to inhibit factor Xa to a target range of 0.35 to 0.7 U/ml.
Low-molecular-weight heparin (150 to 200 U/kg subcutaneously every six to eight hours) is an alternative to unfractionated heparin and is starting to be used to treat thromboembolic disease in veterinary medicine. Low-molecular-weight heparins have better subcutaneous bioavailability, are better able to inhibit factor Xa, and likely can be given at a decreased frequency compared with unfractionated heparin. No clinical studies have been done to determine whether this drug is effective in preventing thromboembolism in patients with IMHA. Measuring anti-Xa activity is necessary to monitor the anticoagulant effects of this drug.
The use of ultra low-dose aspirin (0.5 mg/kg orally once daily) in addition to immunosuppressive medications has shown clear promise in canine patients. The beneficial effects of aspirin are thought to be from vasodilation and modulation of platelet aggregation. One large study in dogs treated with glucocorticoids and azathioprine compared the use of ultra low-dose aspirin, unfractionated heparin, and a combination of these two medications.14 The results demonstrated that the patients treated with aspirin had significantly longer survival times than patients treated with unfractionated heparin alone. In this study, the use of aspirin was not associated with any adverse clinical effects, even when used with high-dose glucocorticoids.
Supportive care
Aggressive, detail-oriented supportive care is a critical factor for the successful treatment of IMHA. Timely recognition and treatment of an underlying disease can allow immunosuppressive drug therapy to be tapered more quickly. Antibiotic administration while awaiting confirmation of suspected infectious disease can improve the chances of recovery. Thorough diagnostic testing can help detect underlying infectious or neoplastic causes of immune-mediated hemolysis, which if missed would make treatment unlikely to succeed. Finally, the quick detection of complications and the speedy removal of nonessential drugs that can cause an immune reaction can also help improve the chances of survival.
Good nursing care that includes daily intravenous catheter care, proper nutrition, short walks, low-stress handling, and limited phlebotomy can also contribute to a better outcome. Peripheral intravenous catheter placement may be preferable to jugular placement in patients with the potential to develop a coagulopathy. Ancillary treatments and medications such as intravenous fluids, gastric protectants, promotility drugs, and antinausea medications can all support a patient through the initial days of treatment. Intravenous fluids, especially in patients with intravascular hemolysis, may help prevent hemogloblin nephrosis. Removing unnecessary indwelling catheters may reduce the risks of thromboembolism.15
LONG-TERM MANAGEMENT AND PROGNOSIS
A steady or rising hematocrit, increasing reticulocytes, and decreasing spherocytes indicate a positive response to therapy.1 Medication tapering usually does not begin until the hematocrit is normal. Tailor drug tapering to the individual patient. Glucocorticoids can be tapered by about 25% every three to four weeks. If an underlying disease has been addressed or the response to treatment is rapid, drug tapering can occur more quickly. Measure the hematocrit five to seven days after discharge and again after each decrease in drug dosage. Perform a complete blood count, reticulocyte count, blood smear, serum chemistry profile, urinalysis, and urine culture every four to eight weeks as needed to monitor for drug side effects, infections, and disease relapse.
A recent study documented that about three months of therapy was sufficient in dogs successfully treated for IMHA, although a subset of dogs needed longer treatment.3 A written drug-tapering and recheck schedule can be provided for the owners to follow, under the supervision of a veterinarian. After finishing all medications, rechecks should occur quarterly for a year, then biannually.
Relapse of disease has been documented to be roughly 12% to 24%, although different protocols and studies make comparison difficult.3,13 If relapse occurs, reinstitute medications at high dosages and taper more slowly. The mortality associated with IMHA is documented to be between 29% and 70%, with a large percentage of deaths occurring within the first two weeks of diagnosis.3 Predictors of increased mortality in dogs include increased blood urea nitrogen concentrations, decreased platelets, and petechiae at the time of diagnosis. Dogs that survive the first two weeks after diagnosis have a six-month survival rate of 92.5%.3
CATS AND IMHA
In cats, IMHA is most often secondary to feline leukemia virus (FeLV) or Mycoplasma haemofelis (formerly Haemobartonella felis) infection although it can also be seen with other infections (feline infectious peritonitis, Cytauxzoon felis infection), drug therapy (methimazole, propylthiouracil), toxins (onions), and neoplasia (lymphoma).40,41 One study documented no increased risk of IMHA in hospitalized cats given subcutaneous vs. intravenous famotidine.42
If you suspect IMHA in a cat based on history, clinical signs, and routine diagnostic test results, perform a FeLV test. Patients with FeLV infection often respond well initially to treatment but eventually succumb to the disease. Cats with M. haemofelis infection typically have recurrent episodes of hemolytic anemia. These organisms can sometimes be seen on a blood smear, although the best test is a PCR. Treat cats with antibiotics, immunosuppressive therapy, and blood transfusions as needed.1
Similar to dogs, cats with IMHA are initially started on immunosuppressive dosages of glucocorticoids. Little information is available describing the addition of secondary or tertiary immunosuppressive medications in cats with severe IMHA. Possible drugs to add include cyclosporine or cyclophosphamide.41
CONCLUSION
When IMHA is first identified, inform owners that the prognosis is variable, treatment is labor-intensive, hospitalization is expensive, and side effects from medications can be severe. Further, since RBC counts and overall stability are often erratic in these patients, prepare owners for the roller-coaster nature of this disease. Owners also need to be prepared for frequent and costly recheck examinations.
Although IMHA is a serious condition, patients can have a good prognosis if they respond to treatment, tolerate the side effects of medications needed for treatment, and do not succumb to secondary infections or thromboembolism. The identification and treatment of underlying disease, the advent of new immunosuppressive drugs, and good supportive and owner care all contribute to increased survival in patients with IMHA.
ACKNOWLEDGMENTS
Special thanks to Jennifer Neel, DVM, DACVP, assistant professor of clinical pathology, Department of Population Health and Pathobiology, College of Veterinary Medicine, North Carolina State University, for providing the photographs in this article.
Nicole Shaw, DVM, DACVIM
Veterinary Emergency and Referral Group
318 Warren St.
Brooklyn, NY 11201
Karyn Harrell, DVM, DACVIM
Department of Clinical Sciences
College of Veterinary Medicine
North Carolina State University
Raleigh, NC 27606
REFERENCES
1. Giger U. Regenerative anemias caused by blood loss or hemolysis. In: Ettinger SJ, Feldman EC. Textbook of veterinary internal medicine. 6th ed. St Louis, Mo: Elsevier Co, 2005;1886-1907.
2. Barker RN. Anemia associated with immune responses. In: Feldman BF, Zinkl JG, Jain NC, eds. Schalm's veterinary hematology. 5th ed. Philadelphia, Pa: Lippincott, Williams & Wilkins, 2000;169-175, 819-826.
3. Piek CJ, Junius G, Dekker A, et al. Idiopathic immune- mediated hemolytic anemia: treatment outcome and prognostic factors in 149 dogs. J Vet Intern Med 2008;22(2):366-373.
4. Liang MM, Pfeiffer I, Roth T. The major histocompatibility complex and its role in canine immune-mediated hemolytic anemia and thrombocytopenia, in Proceedings. 16th European Coll Vet Intern Med Companion Anim Congress 2006.
5. Calvert CA, Sammarco C, Pickus C. Positive Coombs' test results in two dogs treated with amiodarone. J Am Vet Med Assoc 2000;216(12):1933-1936.
6. Van Israel N, French AT, Wotton PR, et al. Hemolysis associated with patent ductus arteriosis coil embolization in a dog. J Vet Intern Med 2001;15(2):153-156.
7. Barker RN, Elson CJ. Red blood cell glycophorins as B and T-cell antigens in canine autoimmune haemolytic anemia. Vet Immunol Immunopathol 1995;47(3-4):225-238.
8. Barker RN, Gruffydd-Jones TJ, Stokes CR, et al. Autoimmune haemolysis in the dog: relationship between anemia and the levels of red blood cell immunoglobulins and complement measured by enzyme-linked antiglobulin test. Vet Immunol Immunopathol 1992:34(1-2):1-20.
9. Schalm OW. Autoimmune hemolytic anemia in the dog. Canine Pract 1975;2:37-45.
10. Stokol T, Blue JT, French TW. Idiopathic pure red cell aplasia and nonregenerative immune-mediated anemia in dogs: 43 cases (1988-1999). J Am Vet Med Assoc 2000;216(9):1429-1436.
11. McManus PM, Craig LE. Correlation between leukocytosis and necropsy findings in dogs with immune-mediated hemolytic anemia: 34 cases (1994-1999). J Am Vet Med Assoc 2001;218(8):1308-1313.
12. Carr AP, Panciera DL, Kidd L. Prognostic factors for mortality and thromboembolism in canine immune-mediated hemolytic anemia: a retrospective study of 72 dogs. J Vet Intern Med 2002;16(5):504-509.
13. Burgess K, Moore A, Rand W, et al. Treatment of immune-mediated hemolytic anemia in dogs with cyclophosphamide. J Vet Intern Med 2000;14(4):456-462.
14. Weinkle TK, Center SA, Randolph JF, et al. Evaluation of prognostic factors, survival rates, and treatment protocols for immune-mediated hemolytic anemia in dogs: 151 cases (1993-2002). J Am Vet Med Assoc 2005;226(11):1869-1880.
15. Miller E. Immune-mediated hemolytic anemia. In: Kirk's current veterinary therapy XIV. St. Louis, Mo: Saunders Elsevier, 2009;266-271.
16. Jackson ML, Kruth SA. Immune-mediated hemolytic anemia and thrombocytopenia in the dog: a retrospective study of 55 cases from 1979 through 1983 at the Western College of Veterinary Medicine. Can Vet J 1985;26(8):245-250.
17. Morley P, Mathes M, Guth A, et al. Anti-erythrocyte antibodies and disease associations in anemic and nonanemic dogs. J Vet Intern Med 2008;22(4):886-892.
18. Boozer L, Macintire D. Babesia gibsoni: An emerging pathogen in dogs. Compend Contin Educ Pract Vet 2005;27(1):33-41.
19. Macintire DK, Boudreaux MK, West GD, et al. Babesia gibsoni infection among dogs in the southeastern United States. J Am Vet Med Assoc 2002;220(3):325-329.
20. Stegeman JR, Birkenheuer AJ, Kruger JM, et al. Transfusion-associated Babesia gibsoni infection in a dog. J Am Vet Med Assoc 2003;222(7):959-963.
21. Birkenheuer AJ, Correa MT, Levy MG, et al. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000-2003). J Am Vet Med Assoc 2005;227(6):942-947.
22. Neer MN. Ehrlichiosis. In: Greene CE, ed. Infectious diseases of the dog and cat. 2nd ed. Philadelphia, Pa: WB Saunders, 1998;139-147.
23. Strickland KN. Canine and feline caval syndrome. Clin Tech Small Anim Pract 1998;13(2):88-95.
24. McCullough S. Immune-mediated hemolytic anemia: understanding the nemesis. Vet Clin North Am Small Anim Pract 2003;33(6):1295-1315.
25. Scott-Moncrieff JC, Treadwell NG, McCullough SM, et al. Hemostatic abnormalities with primary immune-mediated hemolytic anemia. J Am Anim Hosp Assoc 2001;37(3):220-227.
26. Day MJ. Systemic lupus erythematosus. In: Feldman BF, Zinkl JG, Jain NC, eds. Schalm's veterinary hematology. 5th ed. Philadelphia, Pa: Lippincott, Williams & Wilkins, 2000;819-826.
27. Scott-Moncrieff JC. Immune-mediated hemolytic anemia: Treatment. In: IMHA: New perspectives on a challenging disease. Thomson Veterinary Healthcare Communications, Lenexa, Kan, 2002:7-11. In: Vet Med 97(10). Sponsored insert.
28. Reimer ME, Troy GC, Warnick LD. Immune-mediated hemolytic anemia: 70 cases (1988-1996). J Am Anim Hosp Assoc 1999;35(5):384-391.
29. Grundy SA, Barton C. Influence of drug treatment on survival of dogs with immune-mediated hemolytic anemia: 88 cases (1989-1999). J Am Vet Med Assoc 2001;218(4):543-546.
30. Mason N, Duval D, Shofer FS, et al. Cyclophosphamide exerts no beneficial effect over prednisone alone in the initial treatment of acute immune-mediated hemolytic anemia in dogs: a randomized controlled clinical trial. J Vet Intern Med 2003;17(2):206-212.
31. Gregory CR. Immunosuppressive agents. In: Kirk's current veterinary therapy XIII small animal practice. Philadelphia, Pa: WB Saunders Co, 2000;509-513.
32. Nielsen L, Niessen S, Ramsay IK, et al. The use of mycophenolate mofetil in eight dogs with idiopathic immune mediated haemolytic anaemia, in Proceedings. European Coll Vet Intern Med Companion Anim Congress 2005.
33. Miller E. The use of danazol in the therapy of immune-mediated disease of dogs. Semin Vet Med Surg (Small Anim) 1997;12(3):167-169.
34. White HL, O'Toole TE, Rozanski EA, et al. Early treatment of canine immune-mediated hemolytic anemia with intravenous immunoglobulin: 11 cases (1998-2001) (abst). J Vet Intern Med 2002;16(3):318.
35. Mathes M, Jordan M, Dow S. Evaluation of liposomal clodronate in experimental spontaneous autoimmune hemolytic anemia in dogs. Exp Hematol 2006;34(10):1393-1402.
36. Lunn KF. Liposomal clodronate as a novel treatment for immune-mediated hemolytic anemia in dogs. Clinical Trials and Research Projects, Colorado State University, Fort Collins, CO. Personal communication, October 2008.
37. Toll, J, Aronsohn M. Prospective evaluation of medical therapy with or without early splenectomy for treatment of severe immune-mediated hemolytic anemia in the dog, in Proceedings, Am Coll Vet Intern Med 2003.
38. Thompson MF, Scott-Moncreiff JC, Brooks MB. Effect of a single plasma transfusion on thromboembolism in 13 dogs with primary immune-mediated hemolytic anemia. J Am Anim Hosp Assoc 2004;40(6):446-454.
39. Breuhl EL, Scott-Moncrieff C, Brooks M. A prospective study of unfractionated heparin therapy to prevent thrombosis in canine immune-mediated hemolytic anemia, in Proceedings. Am Coll Vet Intern Med 2005.
40. Birkenheuer AJ, Le JA, Valenzisi AM, et al. Cytauxzoon felis infection in cats in the mid-Atlantic states: 34 cases (1998-2004). J Am Vet Med Assoc 2006;228(4):568-571.
41. Kohn B, Weingart C, Eckmann V, et al. Primary immune-mediated hemolytic anemia in 19 cats: diagnosis, therapy, and outcome (1998-2004). J Vet Intern Med 2006;20(1):159-166.
42. de Brito Galvao JF, Trepanier LA. Risk of hemolytic anemia with intravenous administration of famotidine to hospitalized cats. J Vet Intern Med 2008;22(2):325-329.
43. Noble SJ, Armstrong PJ. Bee sting envenomation in secondary immune-mediated hemolytic anemia in two dogs. J Am Vet Med Assoc 1999;214(7):1026-1027.
44. Trepanier L. Idiosyncratic toxicity associated with potentiated sulfonamides in the dog. J Vet Pharmacol Ther 2004:27(3):129-138.
45. Bloom JC, Thiem PA, Sellers TS, et al. Cephalosporin-induced immune mediated cytopenia in the dog: demonstration of erythrocyte-, neutrophil-, and platelet- associated IgG following treatment with cefazedone. Am J Hematol 1988:28(2):71-78.
46. Peterson M. Hyperthyroidism. In: Ettinger SJ, Feldman EC, eds. Textbook of veterinary internal medicine. 5th ed. St. Louis, Mo: Elsevier Co, 2000;1410-1411.
47. EC Feldman, RW Nelson. Feline hyperthyroidism (thyrotoxicosis). Canine and feline endocrinology and reproduction. 3rd ed. St. Louis, Mo: Elsevier Co, 2004;152-218.
48. Bexfield NH, Villiers EJ, Herrtage ME. Immune-mediated haemolytic anemia and thrombocytopenia associated with Anaplasma phagocytophilum in a dog. J Small Anim Pract 2005;46(11):543-548.