Intracavitary and intralesional chemotherapy in dogs and cats
In well-selected cases, these localized chemotherapies have shown promise.
Next >
The intent of chemotherapy in the treatment of cancer is to target rapidly dividing cells in order to kill as many neoplastic cells as possible. However, this mechanism of action can also severely deplete normal cells when chemotherapy agents are given systemically, resulting in potentially serious side effects.
The premise behind intracavitary and intralesional chemotherapy is to expose tumors to higher concentrations of chemotherapy agents while reducing drug exposure to the rest of the body. These techniques, particularly intralesional therapy, are not considered standard of care in veterinary oncology. However, some cases do not lend themselves to further surgery, and many regions do not have radiation therapy services. In those instances, these techniques might be alternatives to preserve patient comfort, function, and survivability. As such, they warrant further studies to better define their safety, efficacy, and applications for use.
INTRACAVITARY CHEMOTHERAPY
The materials needed to perform intracavitary chemotherapy are present in most practices (Figure 1 and Table 1). The rationale behind intracavitary chemotherapy is to expose neoplastic cells within a body cavity to concentrations of a drug higher than those in plasma to provide higher kill rates of neoplastic cells than would be achievable with intravenous chemotherapy.1 For this treatment goal to be achieved, several requirements for the type of cancer and the selected drug must be met.
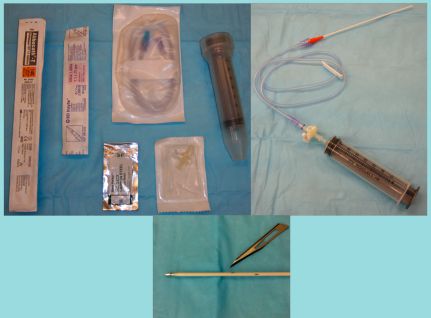
Figure 1. Materials needed to perform intracavitary chemotherapy are inexpensive and can be modified for use from standard hospital materials (Panel A). The three-way stopcock is connected to the syringe for draining fluid from the body cavity with an extension set between the stopcock and catheter (Panel B). Fenestrations in the catheter should be made carefully with a No. 11 blade (Panel C). The fenestrations should not extend more than one-fourth of the total diameter of the catheter and should be smooth. Larger fenestrations or those with rough edges can lead to fracture of the catheter in the body cavity. Two to four fenestrations should be created in the distal half of the catheter.
TABLE 1
Materials used for intrapleural or intraperitoneal chemotherapy administration
• Clippers
• Surgical scrub solution
• Local anesthetic
• 14- to 18-ga catheter
• No. 11 blade
• 30- to 60-ml syringe
• Sterile gloves
• Extension set
• Three-way stopcock
• Collection vessel for fluid drained
• Personal protective equipment for chemotherapy administration
• Barrier pad to place under patient and chemotherapy administration set
• Chemotherapy drug diluted to appropriate volume
• 10 ml of 0.9% saline flush
Tumor type
The tumor type is an important consideration since chemotherapy drugs that are delivered intracavitarily have been shown to penetrate about 3 mm into tissues.2 This means that they are unlikely to be useful in the case of bulky disease or disease deep within organs, but rather are better suited to small tumors that are widely spread on serosal surfaces.3 Alternatively, intracavitary chemotherapy can be used after surgical resection of a tumor to control residual microscopic disease and prevent micrometastasis to the serosa within the cavity.3
These two main applications require that the tumor spreads through serosal seeding rather than through the lymphatics or blood vessels and that the tumor is confined to a body cavity (i.e. no distant metastasis is present).1 This means that intracavitary chemotherapy is best suited to the treatment of carcinomatosis, sarcomatosis, or mesothelioma, as these result in disseminated disease through direct seeding of either the pleural or peritoneal space.2 These qualifications emphasize the need for careful case selection.
Drug type
The drug used also must fulfill several requirements before it should be considered for intracavitary use. The main requirement is that there should be evidence of increased effectiveness if the drug reaches higher concentrations or persists for a longer period.1
Drugs used intracavitarily also should not cause significant damage to the lining of the body cavity into which they are infused. If a drug has the potential to damage the peritoneum or pleura when administered intracavitarily, the dose that can be administered will be limited by the degree of this damage.1 However, if the drug causes little irritation of the serosal surfaces, then it could be given at much higher dosages until the concentration in circulation results in systemic toxicities.1
Cisplatin
Cisplatin is a drug that falls into the category of causing little irritation of the serosal surfaces, in that it is not associated with serosal inflammation and can thus be administered at full intravenous dosage when given by the intracavitary route.1 It should be noted that cisplatin should never be used in cats because it results in fatal pulmonary edema. In people, intracavitary cisplatin has been used to treat several different types of cancer, including ovarian carcinoma and mesothelioma.3,4 It has been used in combination with surgical debulking or with intravenous chemotherapy and has shown good safety and efficacy.3,4
There have been few studies of intracavitary chemotherapy in dogs and cats. The earliest of these studies treated six dogs with either carcinomatosis (three dogs) or mesothelioma (three dogs) that had malignant effusions with intracavitary cisplatin.5 The treatments were scheduled every four weeks, and dogs received from one to six doses (see Table 2 for the procedure followed). Although one dog died of disseminated intravascular coagulation (DIC) 10 days after surgery and intraperitoneal cisplatin administration, all other dogs had resolution of their effusions after intracavitary cisplatin treatment. Of these five dogs, one was lost to follow-up after 129 days, and the other four had prolonged responses and survival times ranging from 306 days to more than 807 days. Given the traditionally poor prognosis for dogs with mesothelioma or carcinomatosis, these results are promising, although the broad clinical applicability is limited by the small number in the report.
The intracavitary cisplatin was well-tolerated, with vomiting being the main sign of toxicosis (in seven of 18 treatments). It is likely that the vomiting seen in this study could be managed by administering maropitant, metoclopramide, ondansetron, or dolasetron as an antiemetic. Because the origin of DIC was unknown in the dog that died 10 days after treatment, it is unclear whether this was a toxic effect of the chemotherapy or due to the cancer itself.
TABLE 2
Steps used to administer intracavitary chemotherapy
- The administration area is clipped-the periumbilical region for intraperitoneal administration or the lateral mid-dorsal thorax over the seventh to ninth rib for intrathoracic administration.
- Aseptic surgical preparation is performed.
- A regional nerve block is administered with an appropriate dose of lidocaine.
- A catheter is prepared by cutting two to four small fenestrations using a No. 11 blade. The fenestrations should be no larger than one-quarter of the circumference of the catheter to avoid catheter fragmentation after insertion (see Figure 1 for an example).
- An extension set is attached to a three-way stopcock and syringe.
- If an attempt to withdraw fluid from the space will not be made, the extension set is flushed with 0.9% saline solution.
- The patient should be placed in comfortable lateral recumbency.
- A barrier drape is placed under the patient, below the catheter insertion point.
- A small stab incision is made through the skin, caudal to the umbilicus or over the lateral mid-dorsal thorax.
- The catheter is introduced past the parietal pleural or peritoneal surface, advanced over the stylet, and then immediately attached to the extension set, three-way stopcock, and syringe combination.
- Fluid is withdrawn as necessary to make the patient comfortable and to allow comfortable instillation of the chemotherapy drug.
- The chemotherapy drug is infused slowly over five to 10 minutes.
- The catheter is flushed with at least 10 ml of 0.9% saline solution to clear the line of the chemotherapy drug.
- The catheter is withdrawn carefully to avoid spilling any liquid. Gentle pressure is applied briefly to the puncture site to prevent bleeding.
- The catheter and barrier drape are discarded with the chemotherapy waste.
Carboplatin and mitoxantrone
Both carboplatin and mitoxantrone have also been reported to have some efficacy administered by intracavitary infusion.2 A study evaluated the use of intracavitary mitoxantrone and carboplatin in the treatment of dogs with carcinomatosis, sarcomatosis, or mesothelioma.2 This study compared seven untreated dogs to 12 dogs treated with intracavitary mitoxantrone, intracavitary carboplatin, or both drugs given on an alternating basis. They found a statistically significant improvement in survival in the treated group, with a median survival time of 332 days vs. 25 days in the untreated group. While four of the 12 treated dogs received intravenous chemotherapy in addition to intracavitary chemotherapy, the clinically significant improvement in the treated group nonetheless provides supportive evidence for the benefit of intracavitary chemotherapy.
The protocol was generally well-tolerated. While five dogs developed either Grade 2 gastrointestinal toxicity or Grade 1 bone marrow toxicity (or both, in the case of two dogs), none of the toxic effects were severe enough to cause a dose reduction or treatment delay. Another interesting finding of this study was that although the presence of effusion negatively impacted the survival time in untreated dogs, it did not affect the survival time in treated dogs. This gives further evidence that intracavitary chemotherapy can be used to treat malignant effusions, which otherwise carry a very poor prognosis.
Doxorubicin
Although the above studies show some promise for the use of intracavitary chemotherapy in the treatment of carcinomatosis, sarcomatosis, and mesothelioma, another study that evaluated intracavitary liposomal-encapsulated doxorubicin to treat hemangiosarcoma had less promising results.6 Dogs with splenic hemangiosarcoma were treated with intracavitary liposomal-encapsulated doxorubicin every three weeks for a total of four treatments. The pegylated liposomal form of doxorubicin was administered with the intent to prolong the half-life and maintain a higher intraperitoneal concentration for a longer period.
Of 14 treated dogs, 12 died of hemangiosarcoma, all of which had hepatic metastasis and hemoabdomen at necropsy. The other two dogs died of unrelated causes. The median survival time of 131 days was not apparently better than historical reports achieved with traditional chemotherapy, although dogs treated with intracavitary doxorubicin had fewer serosal metastases at necropsy. The authors concluded that since the most problematic metastases in dogs with splenic hemangiosarcoma were to the liver, decreasing the serosal metastases did not have a significant impact on survival.
INTRALESIONAL CHEMOTHERAPY
Intralesional chemotherapy is generally administered in surface masses located in the skin and subcutis. Masses located inside cavities, such as the nose or body cavities, are difficult to inject with confidence, as leaking of drug is hard to detect. The premise behind intralesional chemotherapy is much the same as for intracavitary chemotherapy-increasing the concentration of the drug at the tumor site improves the response rates to chemotherapy. Like intracavitary chemotherapy, there is the possibility of achieving a drug concentration in the tumor that exceeds what would be tolerable systemically while simultaneously avoiding systemic side effects. Tumor types with high metastatic potential, such as hemangiosarcoma, are generally poor candidates for this therapy as a sole approach.
Intralesional chemotherapy has not been compared with standard surgical and radiation therapy management of any tumor type, and it is probably an inferior treatment option in most cases. Further, compared with intracavitary and intravenous chemotherapy, intralesional therapy has a much higher likelihood of human exposure during and after administration. Appropriate precautions should be taken, as discussed briefly in the sidebar “Chemotherapy safety” on the last page of this article (page 9).
Soft tissue sarcomas
Veterinary intralesional chemotherapy has been evaluated most frequently in the treatment of canine soft tissue sarcomas. Soft tissue sarcomas of dogs are locally aggressive tumors that are often difficult to completely excise, resulting in recurrence rates as high as 62%.7 Standard therapy is aggressive excision, often followed by radiation therapy when excision is incomplete.8 In regions without access to radiation therapy or in cases in which the cost of radiation is prohibitive, intralesional chemotherapy may be an attractive option. It is less costly than full-course radiation treatment and does not require repeated anesthetic episodes.9
Studies involving chemotherapy implants after incomplete resection have had complication rates too high for this method to be feasible for extensive clinical use. Two studies evaluated cisplatin implants that were placed in the cavity left behind after incomplete resection of soft tissue sarcomas. The earliest of these used a biodegradable polymer sponge (OPLA) as a carrier for cisplatin, and the later study used a polymer gel (Atrigel-QLT Inc.).10,11 While the polymer gel study provided good local control with a local recurrence rate of 16.6%, both studies had unacceptably high complication rates of 60% to 84%. These complications necessitated removal of the implant in most cases.
A different approach to intralesional chemotherapy in soft tissue sarcomas is intra-incisional chemotherapy after incomplete resection. Only one very limited study has been done examining this approach, but its results were promising.9 In this study, six dogs with soft tissue sarcomas and no evidence of metastasis had incomplete resection of their tumors. At least a week after surgery, these dogs received intra-incisional injections of 5-fluorouracil. The drug was injected at multiple sites along the surgical scar (or in the wound bed, in the one surgical site that was not closed primarily). These treatments were continued weekly for a minimum of six treatments.
Of the six treated dogs, two were euthanized-one because of local sarcoma recurrence and the other because of metastasis. The dog with metastasis did not have evidence of local recurrence at necropsy. All other dogs in the study were alive and in complete remission at the time of publication, which ranged from one year to more than three years after starting treatment. The 5-fluorouracil injections were well-tolerated, and no dogs required sedation or local anesthesia for administration. There were no systemic adverse effects noted, and only one dog developed cutaneous hyperpigmentation after treatment, which resolved spontaneously. The good local control rate (83.3% local control at two years) combined with the low incidence of adverse effects makes intra-incisional therapy an option worth exploring for the treatment of soft tissue sarcomas when a second surgery is not feasible and radiation therapy is not available. Note that 5-fluorouracil should never be administered in cats because it results in fatal central neurotoxicity.
Osteosarcoma
Intralesional chemotherapy has been used in an attempt to prevent local recurrence after local excision of osteosarcomas without amputation.12 In this study, 80 dogs underwent limb-sparing surgery for treatment of osteosarcoma, and an implant was placed in the cavity left by tumor resection. In a control group of 40 dogs, a nonimpregnated OPLA implant was placed, while the experimental group of 40 dogs received a cisplatin-impregnated implant (OPLA-Pt). All dogs also received a standard adjuvant chemotherapy protocol of intravenous cisplatin.
Although dogs in the OPLA-Pt group were 50% less likely to have local recurrence than dogs in the control group, this difference was not statistically significant. Infection was a common complication in both groups and occurred in about half of the dogs. However, the authors attributed this complication to the limb-sparing procedure itself rather than to the implant. Systemic cisplatin toxicity was not significantly different between the groups. The authors concluded that if intralesional therapy were optimized, it may be possible to use it in combination with limb-sparing procedures to lower the rate of local recurrence.
Skin cancer
There have been some published data in the human medical literature supporting intralesional therapy as a viable treatment for certain types of skin cancer.13 One study investigated the response of sun-induced squamous cell carcinomas in dogs to treatment with intralesional chemotherapy as a model for similar tumors in people.14 This study used an implant composed of a bovine collagen gel combined with epinephrine (to limit diffusion of the gel from the site) and either 5-fluorouracil alone or 5-fluorouracil and then cisplatin. Thirteen dogs with sun-induced squamous cell carcinoma received injections of the 5-fluorouracil implant weekly until complete response or until no further response was noted. The dogs that failed to have a complete response to 5-fluorouracil then received weekly injections of the cisplatin implant.
Seven of the 13 dogs (54%) had complete responses, with five of these from 5-fluorouracil implants alone and two after treatment with both 5-fluorouracil and cisplatin implants. Of these dogs, only one had local recurrence. All of the dogs without a complete response had a partial response of at least 50% reduction in tumor area. This study was promising in that many of these dogs had received prior therapy, making a complete response rate of over half the dogs a clinically significant result. While local tissue reaction occurred, no systemic toxicities were observed, implying that the intralesional treatment was safe as well as reasonably effective. It must be emphasized that surgical excision and radiation therapy are standard treatment for squamous cell carcinomas.
A carboplatin implant has been evaluated in the treatment of similar squamous cell carcinomas of the nasal planum of cats.15 In one study, 15 cats with squamous cell carcinoma of the nasal planum received four weekly intratumoral injections of an implant of purified sesame oil and carboplatin.
Of these cats' tumors, 73.3% (11/15) had a complete response, and the mean progression-free survival time was 16 months. No systemic toxicity was noted. Mild local toxicity occurred in five cats (local swelling or ulceration), but this was self-limiting. The one-year progression-free survival rate in the treated cats (55.1%) compared favorably with rates achieved with radiation (45.5% to 66.8%). Seven of the cats had local recurrence after treatment; two of these cats received a second course of intralesional carboplatin and had no evidence of disease more than a year after their second round of treatment. This study demonstrated that intralesional carboplatin was a safe and effective treatment for squamous cell carcinomas in cats, with results similar to radiation therapy. A concern worth emphasizing for all intralesional chemotherapy approaches using liquid chemotherapy in the body surface is the safety of the veterinary personnel because of the open mixing and administration of chemotherapy drugs (see the sidebar “Chemotherapy safety” on the last page of this article).
Oral malignant melanoma
Intralesional implants have been evaluated in oral malignant melanomas of dogs.16 A bovine collagen-based implant system was used in which bovine collagen gel and epinephrine were combined with either cisplatin, methotrexate, or carmustine. Twenty dogs with oral malignant melanoma (13 recurrent tumors) were treated with weekly injections of the cisplatin implant, followed by methotrexate implants, and then carmustine if the cisplatin implants did not induce a complete response.
Eleven of 20 dogs (55%) had a complete response, nine after cisplatin treatment alone and two after treatment with cisplatin, methotrexate, and carmustine implants. No systemic toxicities were seen, although local necrosis occurred in 17 of the 20 dogs. The necrosis was associated with the tumor's response to the chemotherapy agent and was treated with surgical débridement. Although the intralesional chemotherapy did not decrease the metastatic rate, it provided reasonable control of local disease, which is an important component in managing this aggressive disease. Systemic therapy, particularly immunotherapy, is still warranted to manage metastatic disease.
Acanthomatous ameloblastoma
Acanthomatous ameloblastoma is a benign but locally invasive tumor of the periodontal ligament. It has a high rate of local recurrence with marginal excision, and it is typically treated with radical surgery (partial maxillectomy or mandibulectomy) or full-course radiation therapy. The recurrence rate with complete surgical excision is less than 5%, but regional resections may be declined by owners for cosmetic or financial reasons.17 Radiation therapy also provides good local control (recurrence rates of 8% to 18%), but it is expensive.17 Radiation therapy also carries the risk of a second malignancy developing within the radiation field, which, in one study, occurred in 3.5% of patients treated for acanthomatous ameloblastoma with radiation.18
In one study, intralesional bleomycin was investigated for the treatment of acanthomatous ameloblastoma in seven dogs with bony invasion.17 One dog had a nonresectable tumor, and this dog's treatment was palliative. Bleomycin was injected into the tumor weekly for up to 16 treatments.
The six dogs treated with a curative intent all had a complete response without recurrence by the time of publication. In most dogs, this response occurred within 30 days. In the dog treated with palliative intent, intralesional bleomycin reduced the tumor by 25% within two weeks. No systemic adverse effects of bleomycin were noted, although local adverse effects were common. In four dogs, wounds with bone exposure formed as a result of treatment and were left to heal by second intention. One of these dogs did not heal and required a partial mandibulectomy. The rate of this complication is notably high in a small, nonrandomized study. Local swelling and tissue irritation were also noted.
Although this mode of treatment had a high rate of local complications, these generally resolved without issue and only required surgical intervention in one case. The 100% complete response rate in those dogs treated with a curative intent offers early evidence of the potential of this treatment option, particularly for owners who do not wish to, or cannot, pursue radical surgery or full-course radiation therapy.
CONCLUSION
Both intracavitary and intralesional chemotherapy are interesting and promising treatment modalities with different applications and should be considered in dogs and cats needing further local care for whom standard care is not available or is not an option. Intracavitary chemotherapy has been effective both in palliating malignant effusions and in prolonging survival times in dogs and cats with mesothelioma and carcinomatosis, diseases typically associated with a poor prognosis.2 Although there is relatively little veterinary research in the field of intracavitary and intralesional chemotherapy, the few existing reports support this approach to therapy in well-selected cases.
REFERENCES
1. Markman M. Intraperitoneal drug delivery of antineoplastics. Drugs 2001;61:1057-1065.
2. Charney SC, Bergman PJ, McKnight JA, et al. Evaluation of intracavitary mitoxantrone and carboplatin for treatment of carcinomatosis, sarcomatosis and mesothelioma, with or without malignant effusions: a retrospective analysis of 12 cases (1997-2002). Vet Comp Oncol 2005;3:171-181.
3. Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. LancetOncol 2003;4:277-283.
4. Tilleman TR, Richards WG, Zellos L, et al. Extrapleural pneumonectomy followed by intracavitary intraoperative hyperthermic cisplatin with pharmacologic cytoprotection for treatment of malignant pleural mesothelioma: a phase II prospective study. J Thorac Cardiovasc Surg 2009;138:405-411.
5. Moore AS, Kirk C, Cardona A. Intracavitary cisplatin chemotherapy experience with six dogs. J Vet Intern Med 1991;5:227-231.
6. Sorenmo K, Samluk M, Clifford C, et al. Clinical and pharmacokinetic characteristics of intracavitary administration of pegylated liposomal encapsulated doxorubicin in dogs with splenic hemangiosarcoma. J Vet Intern Med 2007;21:1347-1354.
7. Bostock DE, Dye MT. Prognosis after surgical excision of canine fibrous connective tissue sarcomas. Vet Pathol 1980;17:581-588.
8. McKnight JA, Mauldin N, McEntee MC, et al. Radiation treatment for incompletely resected soft-tissue sarcomas in dogs. J Am Vet Med Assoc 2000;217:205-210.
9. Marconato L, Comastri S, Lorenzo MR, et al. Postsurgical intra-incisional 5-fluorouracil in dogs with incompletely resected, extremity malignant spindle cell tumors: a pilot study. Vet Comp Oncol 2007;5:239-249.
10. Dernell WS, Withrow SJ, Straw RC, et al. Intracavitary treatment of soft tissue sarcomas in dogs using cisplatin in a biodegradable polymer. Anticancer Res 1997;17:4499-4506.
11. Havlicek M, Straw RS, Langova V, et al. Intra-operative cisplatin for the treatment of canine extremity soft tissue sarcomas. Vet Comp Oncol 2009;7:122-129.
12. Withrow SJ, Liptak JM, Straw RC, et al. Biodegradable cisplatin polymer in limb-sparing surgery for canine osteosarcoma. Ann Surg Oncol 2004;11:705-713.
13. Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol 2010;63:689-702.
14. Kitchell BK, Orenberg EK, Brown DM, et al. Intralesional sustained-release chemotherapy with therapeutic implants for treatment of canine sun-induced squamous cell carcinoma. Eur J Cancer 1995;31A:2093-2098.
15. Théon AP, VanVechten MK, Madewell BR. Intratumoral administration of carboplatin for treatment of squamous cell carcinomas of the nasal plane in cats. Am J Vet Res 1996;57:205-210.
16. Kitchell BE, Brown DM, Luck EE, et al. Intralesional implant for treatment of primary oral malignant melanoma in dogs. J Am Vet Med Assoc 1994;204:229-236.
17. Kelly JM, Belding BA, Schaefer AK. Acanthomatous ameloblastoma in dogs treated with intralesional bleomycin. Vet Comp Oncol 2010;8:81-86.
18. McEntee MC, Page RL, Théon AP, et al. Malignant tumor formation in dogs previously irradiated for acanthomatous epulis. Vet Radiol Ultrasound 2004;45:357-361.
< Back
Chemotherapy safety
Safe handling of chemotherapy agents in the veterinary clinic has been well-described.1 Intracavitary infusion of chemotherapy should be performed with the same safety precautions as intravenous infusion of chemotherapy. Intralesional chemotherapy has an inherently higher risk of administrator and assistant exposure as well as client exposure postinjection. This is because some chemotherapeutic agents are mixed with viscous agents in vials or syringes that are not protected from excessive pressure and leaking, and the preparations often leak out of the injection site, increasing the risk of exposure.
Such risk should be managed by insisting on personal protective equipment, carefully absorbing all leaked fluid at the injection site, and cleaning the site after administration. All materials used in this process should be considered contaminated and should be discarded appropriately. Clients should be told the drug may continue to leak from injection site, they should not handle the injected area without gloves, and they should wash carefully after contact with the animal and its bodily fluids. As with systemic chemotherapy, the animal may continue to excrete chemotherapy metabolites for prolonged periods, so the client should avoid exposure to residues in the urine and feces.
Despite the necessary precautions, in cases in which these procedures are indicated, careful management can result in improved outcomes for the patients compared with inadequate surgery with no adjuvant care.
REFERENCE
1. Allen R, Crump K. Chemotherapy handling, safety, and disposal. InKrump K, Thamm D, eds. Cancer chemotherapy for the veterinary health team. West-Sussex, UK: Wiley-Blackwell, 2011;57-69.
Urinalysis offers a noninasive, rapid screening for canine cancer detection
February 9th 2024This is the first rapid test using urine developed by the Virginia Tech College of Engineering, College of Agriculture and Life Sciences, and the Virginia-Maryland College of Veterinary Medicine
Read More