Managing MRSA, MRSP, and MRSS dermatologic infections in pets
Has one of these resistant infections invaded one of your patients? What should you do now to eliminate the infection? Read on...
In part 1 of this series ("The emergence and prevalence of MRSA, MRSP, and MRSS in pets and people" in the December 2012 issue), we took a look at how methicillin-resistant Staphylococcus aureus (MRSA), methicillin-resistant Staphylococcus pseudintermedius (MRSP), and methicillin-resistant Staphylococcus schleiferi (MRSS) strains have become an increasing problem and the risk factors for infecton with a resistant staphylococcal strain. Here is what to do if you think one of these stubborn strains has taken hold in one of your patients.
DIAGNOSIS
Clinical presentation
Although some of the photos in this article show dramatic lesions, it is important to remember that in most cases methicillin-resistant staphylococcal infections in animals present no differently than methicillin-susceptible infections (Figure 1). Clinical signs of superficial pyoderma include papules, pustules, crusts, scaling, erythema, and hair loss. Patients with deep pyoderma may have nodules or bullae, thick crusts, or ulcerated areas.

Figure 1. An atopic Lhasa apso with a spreading superficial pyoderma caused by MRSP.
A methicillin-resistant infection should be suspected (Figures 2 & 3) whenever there is poor response to empiric antibiotics, especially if a patient has a history of treatment with multiple prior antibiotics or a previous methicillin-resistant infection. Cytologic examination of lesions should be performed to document the presence of bacteria and to aid in interpretation of culture results. Prompt sample submission for bacterial culture and sensitivity testing should be performed rather than an empiric antibiotic change.1

Figure 2. Acral lick dermatitis in an atopic dog, omplicated by deep infection with MRSP.
Culture
Methicillin-resistant infections are diagnosed by bacterial culture and antimicrobial susceptibility testing of appropriate samples submitted to a veterinary reference laboratory. Depending on the clinical presentation of the infection, culture samples can be obtained by sampling otic exudate or an intact pustule with a culture swab, by rubbing a sterile saline-moistened culture swab under crusts or scaly rims of epidermal collarettes, or by obtaining a punch biopsy of a lesion for macerated tissue culture (especially recommended for deep pyoderma).

Figure 3. An atopic Labrador retriever with methicillin-resistant blepharitis.
Here are a few tips to keep in mind once you receive a result from your laboratory:
- If a methicillin-resistant infection is identified, and if the laboratory does not automatically test sensitivities to chloramphenicol, amikacin, and doxycyline, call the laboratory to make sure these antibiotics are added to the sensitivity panel.
- If a coagulase-positive Staphylococcus species is identified but not speciated, call the laboratory to make sure this essential test is done.
- If a coagulase-negative Staphylococcus species is cultured as the only organism from a suspected resistant infection but is not speciated or tested for antibiotic sensitivities, call the laboratory and request that the bacteria be fully speciated and that an antibiotic sensitivity panel be performed.
TREATMENT
Advice to owners
Advise owners of pets with MRSA of the potential for zoonotic transmission (Figures 4A & 4B). Although the risk of clinical disease is probably low for immunocompetent people, reports of community-acquired MRSA in immunocompetent people are on the rise.2 Additionally, the concern for transmission is greater if a pet is exposed to immunosuppressed people or when household members are in contact with higher-risk people (i.e. healthcare workers).3 Involvement of physicians in these cases is prudent because veterinarians should not make specific recommendations for preventing or diagnosing disease in people.

Figure 4A. A cat with methicillin-resistant deep pyoderma (MRSA) secondary to facial pruritus and self trauma due to food allergy. Note that the owner is not wearing gloves and has been topically treating this wound for several months.
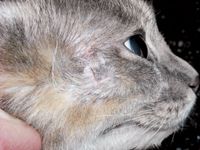
Figure 4B. After six weeks of systemic antibiotics based on bacterial culture and sensitivity testing, the wound has completely healed.
Also advise owners of pets with MRSP and MRSA infections that, for most pets, the prognosis for cure is good with appropriate therapy and monitoring and, in cases of recurrent skin or ear infections, if the underlying cause is identified and addressed (Figure 5).4,5
Colonized animals
As discussed in last month's article, some animals are not infected with but instead are colonized with—or are carriers of—methicillin-resistant staphylococcal strains. Management recommendations for pets colonized by methicillin-resistant staphylococcal bacteria are unclear, but most references recommend practicing good hygiene (washing hands or using hand sanitizer frequently after touching the pet, not allowing a pet to lick or sniff at people, frequent washing and disinfection of pet bedding and housing surfaces) and isolating colonized or infected pets from immunocompromised people and pets.

Figure 5. An atopic dachshund with lichenification and infection of the ventral tail caused by MRSP.
Systemic antibiotics are not recommended to eliminate colonization. Topical antimicrobial shampoos and conditioners (i.e. those containing chlorhexidine) may be helpful but have not been specifically studied in animals. In one study in people, chlorhexidine bathing and intranasal mupirocin application in a 16-bed intensive care unit caused a 48% decrease in MRSA colonization and infection.6 However, topical use of mupirocin in the nasal cavity is unlikely to be successful as monotherapy in MRSA-colonized pets—as studies have shown identical MRSA carriage from the nares, mouth, anus, groin, and head.7
Mucosal carriage of S. pseudintermedius was shown to be significantly decreased in healthy dogs when fusidic acid was applied to the eyes, nostrils, anus, and vulva.8 However, in the absence of any controlled studies on spontaneous decolonization and long-term efficacy of antibacterial therapy for decolonization, it remains controversial whether decolonization of animals is necessary or warranted. If this measure is under consideration, it should involve collaboration between and advice from veterinary and medical infection-control experts and would need to include all in-contact people, in-contact animals, and their environments.1
Infected animals
Routine infections. Localized methicillin-resistant infections may be treated with topical medications such as chlorhexidine sprays or flushes, fusidic acid, or mupirocin applied twice daily until resolution. In cases of generalized pyoderma caused by methicillin-resistant staphylococci, always use aggressive topical antimicrobial therapies; in some cases, frequent (every one to two days) antibacterial (i.e. chlorhexidine) shampoos or conditioners and twice-daily antibacterial sprays (chlorhexidine, hypochlorous acid [Vetericyn—Innovacyn Inc.]) can resolve infection. A recent study comparing in vitro efficacy of antimicrobial shampoos found chlorhexidine to be superior in killing bacteria compared with benzoyl peroxide, ethyl lactate, and chloroxylenol.9
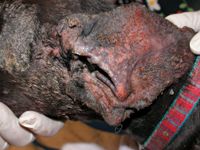
Figure 6A & 6B. Pemphigus foliaceus complicated by a severe MRSP pyoderma and otitis.
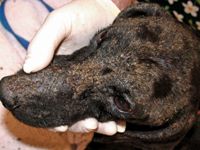
Figure 6B.
Refractory or severe infections. In refractory or severe pyoderma cases, systemic antibiotic therapy is used in combination with topical therapies (Figures 6A & 6B). Because of the variability of methicillin-resistant isolates, antibiotic choice should always be based on in vitro antibiotic susceptibility testing.10 Never treat methicillin-resistant infections with beta-lactam antibiotics (penicillins and cephalosporins), even if in vitro testing implies susceptibility. This is because methicillin resistance means resistance to all beta-lactams, but laboratory reporting errors can occur and erroneously imply sensitivity where it does not exist.11 For superficial pyoderma (whether methicillin susceptible or methicillin resistant), antibiotics are required for at least three weeks (one week beyond complete healing); for deep pyoderma, antibiotics may be needed for four to eight weeks or longer (or two to three weeks beyond complete healing) (Figures 7A & 7B).
Rechecks every two to four weeks are important to evaluate response to treatment, make treatment modifications if needed, and evaluate when antimicrobial therapy can be stopped. Depending on the bacterial strain, antibiotics that may be effective in methicillin-resistant infections include
- Chloramphenicol
- Aminoglycosides
- Potentiated sulfonamides
- Clindamycin (only use if sensitivity to erythromycin is also indicated or a test for inducible clindamycin resistance has been performed)
- Doxycycline (but if sensitivity testing indicates resistance to tetracycline, then doxycycline may not be effective in vivo even if in vitro sensitivity is indicated1 )
- Minocycline
- Rifampin
- Fluoroquinolones

Figure 7A & 7B. Deep ulcers and draining lesions caused by immune mediated panniculitis complicated by MRSP.

Figure 7B.
Use of fluoroquinolones for the treatment of methicillin-resistant pyoderma is not recommended except as a last resort since, with the exception of moxifloxacin, they have the potential to select for high-level methicillin-resistant mutants (which are not only resistant to fluoroquinolones but also to other antibiotics).12,13 If fluoroquinolones are used, then veterinary-labeled products (which have near complete bioavailability)14 are recommended to be used at the high end of the label dose range.15
Use of ciprofloxacin may lead to treatment failure because of inconsistent absorption of this drug in dogs (in one study the oral absorption of ciprofloxacin in dogs varied from 28% to 98%),16 thus it is not recommended. Additionally, the use of vancomycin (administered intravenously only since oral vancomycin is not systemically absorbed) or linezolid for treating methicillin-resistant infections in animals is controversial and not recommended, as these drugs are often the last resort in human medicine.1
Finally, drugs for treating MRSA in human medicine include the streptogramins (quinupristin and dalfopristin), daptomycin, tigecycline, and ceftaroline fosamil; all are administered intravenously, and their use in animals has not been reported.17
Environmental control
Pets treated for a methicillin-resistant infection in a hospital environment must be treated as infectious and isolated from the general hospital population. Do not allow outpatients with suspected or known methicillin-resistant infections to contact other patients in the waiting room, but immediately usher them into an examination room. Use gloves and gowns or dedicated laboratory coats when handling the animals or any in-contact items such as bowls or bandages. Pens and stethoscopes must also be dedicated to the patient. Use disposable thermometer covers, or discard digital thermometers after the animal is discharged.
In people, the most critical step for reducing MRSA transmission is hand hygiene18 ; frequent hand washing was shown to reduce MRSA colonization in equine veterinarians,19 and the same likely applies to small-animal veterinarians. Perform hand hygiene, whether done by hand washing or alcohol-based hand sanitizers, before patient contact, before aseptic procedures, after contamination of the hands, after removing gloves, and after patient contact.20 MRSA can survive up to months on inanimate surfaces, depending on environmental conditions,21 and long-term survival of MRSP is also likely, as MRSP was isolated over a six-month period from household environmental sites and, in some households, beyond resolution of MRSP infection in the pet.22
After discharge, just as with any patient, disinfect all examination room tables, floors, door and sink handles, light switches, scale surfaces, cage items, and medical equipment used on animals with methicillin-resistant infections. Use appropriately diluted disinfectants allowed to contact surfaces for the time listed on the product label (typically five to 10 minutes or longer), after removal of any organic debris that could potentially inactivate disinfectants. Staphylococci, including methicillin-resistant staphylococci, are susceptible to most commonly used disinfectants.20
The importance of environmental cleaning was highlighted by the finding that MRSA carriage in a kennel of rescue dogs resolved spontaneously with regular kennel cleaning alone.23 The development of a good general infection control program in the hospital (rather than focusing solely on methicillin-resistant staphylococci) is probably the most important factor for reducing methicillin-resistant staphylococcal transmission20 (see "Avoid the spread: Methicillin-resistant staphylococcal infection control" below in this article for helpful guidelines).
CONCLUSION
Methicillin-resistant infections in pets are an increasing problem in veterinary medicine and are driven by antibiotic pressure. In patients with recurrent skin and ear infections, evaluation and treatment of the underlying disease is essential to reduce the need for antibiotic treatment of secondary infections. Increased use of topical antiseptics, basing treatment decisions on bacterial culture and antimicrobial susceptibility testing as much as possible, and clearly communicating with clients about the need for full treatment compliance may help reduce selection pressure.20 Additionally, veterinarians must ensure, via frequent hand sanitation and effective hospital infection control programs, that the risk of interpatient spread of infection or colonization is minimized. Also see Checklist: Environmental control of infectious disease to download a list of measures you can take to precent the spread of infectious disease in your practice.
GUIDELINES
Avoid the spread: Methicillin-resistant staphylococcal infection control
Useful recommendations for methicillin-resistant infection management can be found in the following online resources:
• Information sheets for pet owners from the University of Guelph Centre for Public Health & Zoonoses
• Information on MRSA infections from the Centers for Disease Control and Prevention

Kimberly S. Coyner, DVM, DACVD
Dermatology Clinic for Animals of Las Vegas
5231 W. Charleston Blvd.
Las Vegas, NV 89146
REFERENCES
1. Frank LA, Loeffler A. Meticillin-resistant Staphylococcus pseudintermedius: clinical challenge and treatment options. Vet Dermatol 2012; 23(4):283-291.
2. Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community- and health care–associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290(22):2976-2984.
3. Manian F. Asymptomatic nasal carriage of mupirocin-resistant, methicillin-resistant Staphylococcus aureus (MRSA) in a pet dog associated with MRSA infection in household contact. Clin Infect Dis 2003;36(2):e26-28.
4. Faires MC, Traverse M, Tater K, et al. Methicillin-resistant and –susceptible Staphylococcus aureus infections in dogs. Emerg Infect Dis 2010;16(1): 69-75.
5. Bryan J, Frank L, Rohrbach BW, et al. Treatment outcome of dogs with methicillin-resistant and methicillin-susceptible Staphylococcus pseudintermedius pyoderma. Vet Dermatol 2012;23(4):361-368.
6. Ridenour G, Lampen R, Federspiel J, et al. Selective use of intranasal mupirocin and chlorhexidine bathing and the incidence of methicillin-resistant Staphylococcus aureus colonization and infection among intensive care unit patients. Infect Control Hosp Epidemiol 2007;28(10):1155-1161.
7. Griffeth GC, Morris DO, Abraham JL, et al. Screening for skin carriage of methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Vet Dermatol 2008;19(3):142-149.
8. Saijonmaa-Koulumies L, Parsons E, Lloyd DH. Elimination of Staphylococcus intermedius in healthy dogs by topical treatment with fusidic acid. J Small Anim Pract 1998;39(7):341-347.
9. Young, R, Buckley L, McEwan N, et al. Comparative in vitro efficacy of antimicrobial shampoos: a pilot study. Vet Dermatol 2012;23(1):36-40.
10. Bemis DA, Jones RD, Frank LA, et al. Evaluation of susceptibility test breakpoints used to predict mecA-mediated resistance in Staphylococcus pseudintermedius isolated from dogs. J Vet Diagn Invest 2009;21(1):53-58.
11. Deresinski S. Methicillin-resistant Staphylococcus aureus: an evolutionary, epidemiologic, and therapeutic odyssey. Clin Infect Dis 2005;40(4):562-573.
12. Dalhoff A, Schubert S. Dichotomous selection of high-level oxacillin resistance in Staphylococcus aureus by fluoroquinolones. Int J Antimicrob Agents 2010;36(3):216-221.
13. Venezia RA, Domaracki BE, Evans AM, et al. Selection of high-level oxacillin resistance in heteroresistant Staphylococcus aureus by fluoroquinolones exposure. J Antimicrob Chemother 2001;48(3):375-381.
14. Papich MG, Riviere JE. Fluoroquinolone antimicrobial drugs. In: Riviere JE, Papich MG, eds. Veterinary pharmacology and therapeutics, 9th ed. Ames, Iowa: Wiley-Blackwell Publishing, 2009.
15. Aucoin DP. Baytril: historical impact and milestones of veterinary medicine's most successful antimicrobial, in Proceedings. Fourth Intl Baytril Symp, 2009;6-15.
16. Papich MG. Ciprofloxacin pharmacokinetics and oral absorption of generic ciprofloxacin tablets in dogs. Am J Vet Res 2012;73(7):1085-1091.
17. Papich MG. Selection of antibiotics for meticillin-resistant Staphylococcus pseudintermedius: time to revisit some old drugs? Vet Dermatol 2012;23(4):352-360.
18. Pittet D, Hugonnet S, Harbarth S, et al. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Lancet 2000;356(9238):1307-1312.
19. Anderson ME, Lefebvre SL, Weese JS. Evaluation of prevalence and risk factors for methicillin-resistant Staphylococcus aureus colonization in veterinary personnel attending an international equine veterinary conference. Vet Microbiol 2008;129(3-4):410-417.
20. Weese JS. Staphylococcal control in the veterinary hospital. Vet Dermatol 2012;23(4):292-298.
21. Kramer A, Schwebke L, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 2006;6:130.
22. Laarhoven LM, de Heus P, van Luijn J, et al. Longitudinal study on methicillin-resistant Staphylococcus pseudintermedius in households. PLoS One 2011;6(11):e277788.
23. Loeffler A, Pfeiffer DU, Lindsay JA, et al. Lack of transmission of methicillin-resistant Staphylococcus aureus (MRSA) between apparently healthy dogs in a rescue kennel. Vet Microbiol 2010;141(1-2):178-181.