Performing a basic examination in fish
Pet fish are one of the most numerous companion animals in U.S. households, yet few fish owners consult veterinarians about fish disease partly because historically veterinarians have declined to offer them assistance. Yet all veterinarians are trained in pathology, diagnostics, animal husbandry, and pharmacology, so who better to apply the principles of these disciplines to pet fish.
Pet fish are one of the most numerous companion animals in U.S. households, yet few fish owners consult veterinarians about fish disease partly because historically veterinarians have declined to offer them assistance. Yet all veterinarians are trained in pathology, diagnostics, animal husbandry, and pharmacology, so who better to apply the principles of these disciplines to pet fish.
The basic approach to evaluating a pet fish is similar to that of evaluating mammals, birds, reptiles, and amphibians, though fish medicine requires a few unique diagnostic methods, such as performing water quality tests and collecting special samples for wet mount preparations.
EQUIPMENT NEEDED
You can diagnose most fish diseases by using a high-quality microscope that has 10X, 40X, and 100X (oil immersion) objectives.
The ability to test water quality is also important. Inexpensive semiquantitative water quality test kits (e.g. Marine Enterprises International, Baltimore, Md.; Tetra, Blacksburg, Va.) provide a general indication of the various water quality parameters. The more expensive test kits (e.g. Aquaculture Multi-Parameter Test Kit, Model FF-1A or FF-2—Hach, Loveland, Colo.) are more accurate and appropriate for diagnostic testing of water quality. In addition, a number of electronic probes are available for monitoring temperature, pH, dissolved oxygen, chloride, and conductivity (salinity). These probes tend to be expensive and need to be properly calibrated.
Aquariums of various sizes (10 gal for small fish, 150-gal stock tanks for larger fish such as koi), clear buckets (5 and 10 gal), fish nets in various sizes, air pumps with air stones, small filtration systems, a water pump for anesthetic delivery, sodium thiosulfate to remove chlorine from water, and tricaine methanesulfonate (MS-222; Finquel—Argent Chemical Laboratories, Redmond, Wash.) for anesthesia are also needed to care for fish.
TRANSPORTING THE FISH
Before clients bring fish to your hospital, advise them about proper transportation. Fish should be transported in a container filled with water that varies little in quality and temperature from that of the fish's normal habitat. Ask the owner to bring a water sample (at least 120 ml [4 oz]) from the habitat in a separate sealed container for water quality testing. For meaningful results, test the water within one hour of collection. Poor water quality is the most common cause of disease in pet fish, so water quality testing is crucial (see boxed text titled "Identifying and correcting water problems").

Identifying and correcting water problems
HISTORY
To obtain a thorough history, learn about the owner's level of expertise in keeping fish, the current problems a fish is having, any past problems, and the overall husbandry provided. Important husbandry information includes the size of the aquarium or pond; the animal population of the aquatic system; the water temperature, filtration type, and aeration method; and the quality and quantity of light. Abnormal photoperiods, such as prolonged light, can cause chronic stress and add to other stressors that may be affecting the aquatic inhabitants. A photoperiod that matches the natural environment of the animals is best. Full-spectrum lighting is more important to the plants in the aquatic system; however, light intensity should match that of the animal's natural environment, if that information is available. Also ask the owner about the water quality, diet, quarantine practices, and current and previous treatments.
INITIAL OBSERVATIONS
Before performing a hands-on physical examination, it is best to examine the fish in the aquarium or pond, if possible, or in the transport container. Observe the activity and body language of the fish in the water. It is important to know the normal behavior of the species of fish being evaluated. For example, male bettas (Betta splendens) are often kept in small bowls without filtration or temperature control. They normally use the midlevel or upper level of the bowl; however, when the temperature drops too low, they sit on the bottom and are reluctant to move. Also, the foraging behavior of certain fish may be confused with abnormal behavior.
Certain abnormal behaviors, however, are common to all fish. The more important of these abnormal behaviors are
- Coughing—flaring of the operculum followed by rapid closure in an attempt to dislodge an irritant from the gills, which is suggestive of gill disease.
- Flashing—rubbing against objects, which is suggestive of an ectoparasitic infestation.
- Piping—gulping air at the surface, indicating hypoxia due to oxygen-poor water, gill disease, or anemia.
- Circling (controlled) or whirling (uncontrolled)—suggestive of blindness and neurologic disease, respectively.
- Drifting—aimless, unpropelled motion indicative of weakness and imminent death.
- Abnormal posture—floating at the surface, which may suggest disorders such as swim bladder or neurologic disease. Or in the case of goldfish, a lack of fiber in flake food diets often results in poor intestinal motility, resulting in air accumulating in the intestines. This air causes the fish to float abnormally at the surface.
PHYSICAL EXAMINATION AND SAMPLE COLLECTION
After taking the history, observing the fish, and performing water quality testing, perform a hands-on examination. To do this, first capture the fish with soft mesh or plastic nets to minimize trauma. The physical examination should be performed quickly (in less than one minute) and may require sedation or general anesthesia. Tricaine methanesulfonate dissolved in water is commonly used to sedate or anesthetize fish. The response of fish to tricaine varies among species and water quality parameters; however, the general dose for sedation ranges between 8 and 30 ppm using a buffered 10 g/L stock solution.1 Fish placed in the anesthetic bath should be immobilized in less than five minutes if given the proper dosage. You can add more tricaine gradually as needed to obtain the desired level of sedation or anesthesia. Anesthetic recovery occurs when the fish is returned to water containing no tricaine anesthetic.
Use latex surgical gloves without powder to examine the external surfaces and eyes. Examine the fish for abnormal color changes, eye lesions, swelling of the coelomic cavity (dropsy), and musculoskeletal abnormalities. Examine the gills by lifting the operculum. Spread the fins to examine them, and evaluate the oral cavity. Compare ill fish with healthy tank mates. Sick fish frequently exhibit changes in color and appear lighter or darker than their healthy tank mates. Reddened areas on the body are usually associated with hemorrhage. Dropsy can be related to peritonitis, ascites from renal or liver failure, obesity, or neoplasia. Traumatic eye lesions are common in fish. Exophthalmia is also common and frequently associated with infectious diseases. Musculoskeletal abnormalities are associated with nutritional abnormalities or infectious diseases.
Obtain samples for diagnostic tests quickly and safely. Blood for diagnostic sampling can be collected safely from fish more than 3 in (8 cm) long.2 The estimated blood volume of fish is about 5% of the body weight (compared with 10% of the body weight of mammals).3 Because the hemodynamics of fish differs from that of mammals, about 30% to 50% of the total blood volume can be safely collected from healthy fish.4 For example, a 100-g fish has an estimated total blood volume of 5 ml, so a 1.5- to 2.5-ml blood sample can be collected. However, this amount is rarely needed for diagnostic purposes. A safe and easy to remember sample size is 1% of the total body weight; therefore, a 1-ml sample would be collected from a 100-g fish.

Figure 1: Blood collection by caudal venipuncture using the lateral approach in a koi.
Blood can be collected by venipuncture of the caudal vein or artery in most species. The caudal vertebral blood vessels can be approached ventrally or laterally. The ventral approach involves inserting the needle along the ventral midline near the base of the caudal peduncle. A lateral approach to the caudal vertebral vessels is performed by inserting the needle a few millimeters below the lateral line near the base of the caudal peduncle (Figure 1). Direct the needle toward the midline and under the vertebral bodies.
Blood samples are collected for complete blood counts, serum chemistry profiles, and toxicology screens. For the best results, perform hematologic evaluation immediately by using your in-house laboratory, but other testing can be performed by commercial laboratories.

Figure 2: Scraping the skin of a koi to obtain mucus with a microscope slide.
Coelomic fluid can be collected by aspiration in fish with dropsy. The fluid sample is evaluated in the same manner as abdominal fluid in mammals; that is, evaluate cellular and protein content and perform a microbial culture.
Obtain a mucus smear by pressing a clean microscope slide onto the surface of a lesion or by gently scraping the skin with the edge of the slide in a cranial to caudal direction (Figure 2). Examine the mucus smear as a wet mount with a drop of tank water. In general, microscopic evaluation of mucus smears are performed to detect parasites, fungi, and bacteria.

Figure 3: A fin clip biopsy performed on a koi.
Obtain a fin biopsy sample by using fine-tipped scissors to remove a triangular wedge of fin tissue, ideally between the fin rays (Figure 3). Evaluate the sample as a wet mount under a coverslip. Microscopic examinations of fin samples are used to detect ectoparasites, fungi, and bacteria.

Figure 4: A gill biopsy performed by using fine-tipped scissors inserted under the operculum of a koi.
After opening the operculum, obtain a gill biopsy sample by using fine-tipped scissors to remove a few tips of primary gill lamellae (Figure 4), and examine it as a wet mount. Gill biopsy samples are evaluated microscopically to assess gill damage, such as hyperplasia and hypertrophy of epithelial cells and increased mucus production that can compromise gill function (Figure 5). Gill injury commonly occurs with bacterial or parasitic infections or with poor water quality.

Figure 5: A wet mount of a gill biopsy sample revealing focal epithelial hyperplasia and excessive mucus production associated with a trichodinid parasite (100X).
Obtain a fecal sample by inserting a swab or small loop through the vent and into the intestine or by collecting feces as they pass from the fish. A fecal sample is also examined as a wet mount. Microscopic examination of feces is used to detect parasite ova or potentially pathogenic protozoa.
QUARANTINE AND INITIAL TREATMENT
Isolate sick fish, especially those suspected of having an infectious disease, from other fish in a separate aquatic system until they have recovered. Consider a broad-spectrum therapy, such as a prolonged immersion with a low concentration of copper (see boxed text titled "Copper treatment") or formalin, during the quarantine period. Provide specific treatment of fish in quarantine exhibiting parasitic, fungal, or bacterial infections once a diagnosis is made.

Copper treatment
COMMON NONINFECTIOUS DISORDERS IN FISH
Common husbandry-related disorders in fish include toxicities caused by ammonia, nitrites, heavy metals, and poisons introduced into the aquatic system.
Traumatic injury related to capture or aggressive tank mates is another husbandry-related problem.
Lateral line erosion, or hole-in-the-head syndrome, is a condition resulting in ulceration of the skin associated with the sensory tissue on the head and lateral line of a fish (Figure 6). The cause of this condition is unknown, but it improves by addressing husbandry problems such as improving the diet (feeding natural foods) and correcting water quality problems.

Figure 6: Lateral line erosion in a blue tang. Note the mild periocular cutaneous ulcers.
Air embolism, or gas bubble disease, is diagnosed by observing gas bubbles in the fins, gills, and eyes of fish. It is caused by cavitating pumps (usually requires pumps of ½ horsepower or larger) that force oxygen and nitrogen into solution. Cavitation occurs when the pump sucks in air either at the intake (when an air stone is placed near the intake) or through a broken valve.
Malnutrition is another possible contributor to disease. The exact nutritional requirements of most fish are unknown, so an underlying deficiency of certain nutrients may be difficult to detect and may predispose fish to secondary diseases. Conditions such as hepatic lipidosis, emaciation, loss of dorsal musculature, disproportionate bodies (big heads and small bodies), and gastrointestinal disorders (e.g. constipation) are most likely directly related to the diet. Obesity and hepatic lipidosis are associated with a high dietary fat intake. Ideally, fish should be fed a diet identical to their natural diet.
COMMON INFECTIOUS DISORDERS IN FISH
Pet fish are susceptible to bacterial, fungal, and viral infections as well as parasitic infestations. A common husbandry-related cause of infectious disease is failure to quarantine new fish before introducing them to the established system. As a result, outbreaks involving pathogenic organisms occur. New fish should be quarantined in a separate system for four to six weeks before their introduction into an established system with other fish. Monitor fish in quarantine for clinical signs of disease. Consider a broad-spectrum therapy, such as a prolonged immersion with a low concentration of copper (see boxed text titled "Copper treatment") or formalin, as a prophylactic treatment.
Bacterial infections
Bacterial disease is one of the most common causes of aquarium fish mortality. Most pathogenic bacteria are gram-negative aerobes and facultative anaerobes.5,6 Immunosuppression due to stress, poor nutrition, a poor environment, or parasitic infestations often leads to bacterial infections with opportunistic pathogens. Clinical signs may vary but often include lethargy, anorexia, hemorrhages (erythema of the fins, mouth, or vent), cutaneous ulcers, fin rot, ascites, exophthalmia, abnormal posturing, and color change. During postmortem examinations, the kidneys provide the best location for bacterial culture in suspected cases of bacteremia or septicemia. Bacterial cultures are best performed by microbiology laboratories that routinely culture pathogenic bacteria from fish.
Fish with bacterial infections can be treated by placing antibiotics in gelatin diets, giving commercially medicated foods, loading food with the antibiotic, or medicating by stomach tube. Antibiotics can also be delivered by intramuscular or intracoelomic injection or through the water either as a bath (10 to 60 minutes) or prolonged immersion (greater than or equal to 24 hr). Most treatments are empirically derived, but formularies are available to guide antibiotic therapy.1,2,5,6
Fungal infections
Fungal infections, another important cause of disease in fish, are usually the result of immunosuppression associated with poor water quality, stress, and other diseases. Prolonged treatment with antibiotics may also predispose fish to fungal infections. Saprolegniasis is a catch-all term for white, fuzzy mold growth on the skin of fish. Saprolegnia is a genus of water mold that commonly infects fish and their eggs. Immunosuppression, resulting from, for example, a drop in temperature or stress from overcrowding, can predispose fish to such fungal infections. Fish with fungal infections are treated with antifungal agents given orally in food, by injections, or by the water-borne route.1,2,5,6
Viral infections
Fifty-six viruses have been reported in fish.7 Viral diseases are frequently associated with secondary bacterial, fungal, or parasitic infections that may lead to a misdiagnosis.
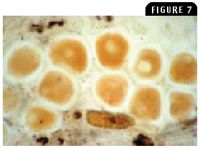
Figure 7: A wet mount of a lymphocystis lesion exhibiting the enlarged virus-infected dermal fibroblasts (100X).
Lymphocystis disease, one of the first viral diseases described in fish, is common to freshwater and marine fish. It is caused by a DNA iridovirus, and infected cells (fibroblasts) increase up to 50,000 times normal size (Figure 7). Advanced lesions exhibit large wartlike tumorous growths on the skin and fins. The disease is transmitted by direct contact and is typically self-limiting, unless growths around the mouth cause starvation. Regression of the lesions may take several months, and affected fish should be isolated from other fish. Keep recovered fish isolated for at least one month to prevent spread of the virus to other fish.
Carp pox, or cyprinid herpesvirus I or Herpesvirus cyprini, is typically a benign self-limiting disease that causes epidermal hyperplasia that appears as superficial, soft, white to gray, waxy or mucoid lesions on the skin and fins of carp and koi. The lesions can persist for several months in affected fish, especially in cooler water temperatures, but resolve when the water temperature warms in the spring. Carp pox can result in systemic disease and high mortality in juvenile cyprinids (less than 2 months of age), such as carp and koi.8
Spring viremia of carp is a reportable viral disease caused by Rhabdovirus carpio that causes high morbidity and mortality in cultivated carp, especially young carp, and related fish such as koi and goldfish. Clinical signs of this viral infection are nonspecific because multiple organs are involved; however, dropsy, darkened skin, and exophthalmia are common.8 Necropsy reveals small hemorrhages throughout the body, ascites, peritonitis, enteritis, and edema of internal organs. Outbreaks of the infection are commonly seen in the spring but can occur whenever the water temperature drops below 64 F (17.8 C).8 A definitive diagnosis is made by virus isolation, or indirect tests such as ELISA and virus neutralization are available to detect the virus in a fish population. There is no treatment for this disease, but keeping the water temperature above 68 F (20 C) helps decrease the mortality. Depopulation of infected fish followed by disinfection (1 part bleach to 10 parts water) of the habitat is recommended to control the disease.
Koi herpesvirus infection is an acute viral disease that can cause high morbidity and mortality in carp and koi. Clinical signs of the viral infection are nonspecific, but severe gill lesions with red and white patches (edema and necrosis), pale raised patches of skin, and sunken eyes are common.9 Affected fish may also exhibit neurologic signs such as erratic swimming and disorientation. Clinical disease commonly appears in a collection of koi and carp during the spring or summer within two to four weeks after introducing new fish to the system or returning from a koi show. The mortality related to koi herpesvirus infection typically occurs in water temperatures between 64 and 81 F (17.8 and 27.2 C). A definitive diagnosis is based on positive results on culture or polymerase chain reaction testing (diagnostic laboratory at the University of Georgia). There is no treatment for koi herpesvirus infection, and surviving fish should be considered carriers. Therefore, depopulation followed by disinfection of the habitat is recommended to control disease.
Parasitic infestations
Parasitic infestations are common in fish. They are diagnosed by wet mount examination of the mucus (from skin), fins, or gills as mentioned above.
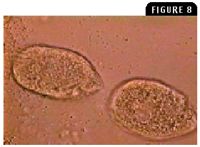
Figure 8: Chilodonella species (40 to 60 µm long) are flattened, oval-to heart-shaped ciliate protozoa that move in a slow circular fashion when viewed in a wet mount (1,000X).
Parasitic protozoa
Ciliate protozoa have a direct life cycle, and most are commensal. However, a few are notoriously pathogenic. Ichthyophthirius multifiliis (known as Ich) in freshwater fish and Cryptocaryon irritans in marine fish are highly pathogenic ectoparasites that feed on host cells. Trichodina species in freshwater and marine fish is another pathogenic ciliate protozoan that can damage the gills and skin when large numbers of the protozoan are present. Chilodonella piscicola and Chilodonella hexasticha in freshwater fish and Brooklynella hostilis in marine fish are ciliate protozoa that cause excessive mucus production and hemorrhage in the gills (Figure 8). Tetrahymena species in freshwater fish and Uronema species in marine fish are normally free-living commensals that become secondary pathogens that are highly invasive and can be found in internal organs (Figure 9). Epistylis species in freshwater fish are ectocommensal ciliate protozoa (Figure 10) that create white tufts on hard surfaces of the fish, such as the fin rays and scales.
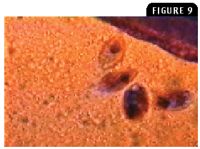
Figure 9: Tetrahymena species (50 to 100 µm long) are pear-shaped ciliate protozoa that are actively motile in a wet mount (100X).
Flagellate protozoa may have direct life cycles; some have resistant cyst stages. The hemoflagellates have indirect life cycles. Ichthyobodo necator (also called Costia necatrix) in freshwater and marine fish attaches to skin and gills, feeding on host cells and causing epithelial hyperplasia and goblet cell destruction. Amyloodinium species is a common dinoflagellate parasite in tropical marine fish that affects both teleost and elasmobranchs, such as sharks and rays. It is detected by identifying the trophonts in wet mount preparations of the skin or gills. Piscinoodinium species, the freshwater counterpart to Amyloodinium species, contains chlorophyll and causes velvet disease, or rust disease, in tropical pet fish. Fish with heavy infestations exhibit a rusty or yellow sheen to the affected skin. Hexamita and Spironucleus species in freshwater and marine fish are flagellate protozoa in the gastrointestinal tract of fish that can cause anorexia, lethargy, and death. Fish exhibiting scant, mucoid feces should be examined for a possible intestinal flagellate infestation. Massive systemic infections, especially with Spironucleus species, are lethal (Figure 11). Other flagellates, such as Cryptobia species, can also be important pathogens. Trypanosome infections are usually asymptomatic, and their pathogenesis is unknown. Trypanosomes are incidental findings in blood films or in imprints of tissues such as the kidneys.
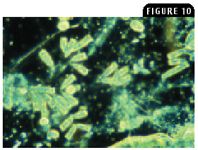
Figure 10: Epistylis species is a stalked bell-shaped ciliate protozoan whose stalks tend to coil during contractions in a wet mount (100X, phase contrast microscopy).
Other protozoal parasites include the microsporidia, such as Plistophora hyphessobryconis in freshwater fish that causes neon tetra disease.10 Myxosporidia species are highly pathogenic, usually intracellular, and involve all organs (Figures 12A & 12B).
Protozoal infestations are usually treated topically with medicated dips or baths. The distinction between a dip and a bath may vary among authors as does the preference for dosages, but in general a dip is exposure to a medicated solution for less than 15 minutes, whereas a bath is for a longer period. Prolonged immersion treatments are those that provide a constant exposure to the medication over several days. Parasitic protozoa can be treated with formalin (0.125 to 0.250 ml 37% formaldehyde/L) as a one-to 60-minute bath; malachite green (0.1 to 0.15 mg/L) as a prolonged immersion; salt (10 to 30 g/L) as a bath up to 30 min (a four-to five-minute salt solution dip for freshwater fish or freshwater dip for marine fish); or formalin (0.02 ml 37% formaldehyde/L) plus malachite green (0.1 mg/L) as a prolonged immersion.1,2 Formalin solutions should not be used if they contain white paraformaldehyde precipitates.1
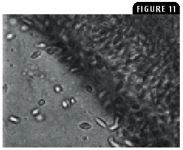
Figure 11: A wet mount teaming with highly motile hexamitid (Hexamita or Spironucleus species) protozoa (5 to 11 µm long) in an intestinal sample from a gourami exhibiting marked weight loss (1,000X).
Trematodes
Monogenean (skin or gill fluke) infestation occurs in both freshwater and marine fish. Monogeneans are ectoparasites that live on skin, gills, and fins. They contain a haptor (attachment organ) and have a direct life cycle. Dactylogyrus species has four points at the anterior end, an anterior sucker, four eyespots, and a haptor with two large hooks surrounded by several small hooklets. Gyrodactylus species has two points at the anterior end, an anterior sucker, no eyespots, and a haptor with two large hooks surrounded by several small hooklets. This monogenean is viviparous with internal embryos containing hooks. Clinical signs associated with monogenean infestation include flashing and skin disorders because of the injury to skin caused by the parasite's attachment and feeding behavior. The hooklets on the haptor penetrate epithelial cells.
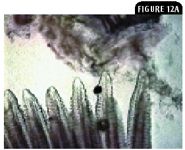
Figure 12A: A gill biopsy from a Corydoras species exhibiting nodules or pseudocysts on the gill filaments (100X).
Monogeneans can be treated by using formalin (0.125 to 0.250 ml 37% formaldehyde/L) as a one-to 60-minute bath; praziquantel (2 mg/L for freshwater fish or 20 mg/L for marine fish) as a one-to three-hour bath; trichlorfon (dimethyl phosphonate; 0.25 to 1 mg/L) as a one-hour bath; or a salt bath (30 to 35 g/L) in freshwater fish.1,2
Digenetic trematodes are endoparasites with an indirect life cycle. The indirect life cycle of digenetic trematodes involves a definitive host that is a fish-eating bird, a first intermediate host that is a snail, and a second intermediate host that is a fish. Adults live in the gastrointestinal tract and have two suckers and a Y-shaped gut. The metacercariae encyst throughout the body of the fish intermediate host. Most fish with encysted metacercariae exhibit no signs of illness; however, those with heavily infested organs may have organ dysfunction. Encysted digeneans are difficult to treat, but praziquantel may reduce their number.2
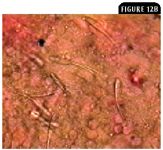
Figure 12B: Spores of Henneguya species from a ruptured pseudocyst on the gill filaments in Figure 12A. This myxozoan, frequently found in wild caught species of Corydoras species, usually causes a self-limiting disease (1,000X).2
Parasitic crustaceans
Parasitic crustaceans include the Branchiura, which are ectoparasites with a dorsoventrally flattened body and prehensile suckers that attach to the bodies of fish.11 An example is Argulus species (fish louse), which has a direct life cycle and causes cutaneous lesions and respiratory distress. It is large enough to be seen grossly, and clients may observe the parasite's movements (Figure 13).
Copepods are another group of parasitic crustaceans with a diversity of body forms with variable appendages. An example is Lernaea species (anchor worm), which is an elongated copepod that embeds its head into the skin of fish, leaving its Y-shaped egg sacs to hang from the fish. These sacs can easily be seen grossly. The anchor worm has a direct life cycle and lives in fresh water.
Organophosphates are the typical treatment for parasitic crustacean infestations. Dichlorvos and trichlorfon are the most commonly used organophosphates for treating fish with parasitic crustacean, monogenean, or leech infestation. Trichlorfon (0.5 to 1 mg/L) as a prolonged immersion or dichlorvos (0.5 to 2 mg/L) as a 30-to 60-minute bath is effective.1,2 Diflubenzuron (Dimilin—PondCare) is a chitin synthesis inhibitor that when used at a dose of 0.01 mg/L as a prolonged immersion treatment can also rid fish of crustacean copepod infestation.

Figure13: Argulus species infestation on the caudal fins of a goldfish. The parasite has a flattened, saucer-shaped appearance.
Terry W. Campbell, MS, DVM, PhD
Department of Clinical Sciences
College of Veterinary Medicine and Biomedical Sciences
Colorado State University
Fort Collins, CO 80523
REFERENCES
1. Lewbart GA. Fish. In: Carpenter JW, ed. Exotic animal formulary. 3rd ed. St. Louis, Mo: Elsevier Saunders, 2005.
2. Noga EJ. Fish disease: diagnosis and treatment. Ames: Iowa State University Press, 2000.
3. Roberts RJ, Ellis AE. The anatomy and physiology of teleosts. In: Roberts RJ, ed. Fish pathology. Philadelphia, Pa: WB Saunders Co, 2001;12-54.
4. Groff JM, Zinkl JG. Hematology and clinical chemistry of cyprinid fish. Vet Clin North Am Exot Anim Pract 1999;2:741-776.
5. Lewbart GA. Medical management of disorders of freshwater fish. Compend Contin Educ Pract Vet 1991;13:969-974.
6. Whitaker BR. Common disorders of marine fish. Compend Contin Educ Pract Vet 1991;13:960-967.
7. Gratzek JB. The science of fish health and management (aquariology series). Morris Plains, NJ: Tetra Press, 1992.
8. McAllister PE. Goldfish, koi, and carp viruses. In: Stoskopf MK, ed. Fish medicine. Philadelphia, Pa: WB Saunders Co, 1993;478-486.
9. Hedrick RP, Gilad O, Yun S, et al. A herpesvirus associated with mass mortality of juvenile and adult koi, a strain of common carp. J Aquatic Anim Health 2000;12:44-57.
10. Tucker CS. Water analysis. In: Stoskopf MK, ed. Fish medicine. Philadelphia, Pa: WB Saunders Co, 1993;166-197.
11. Moe MA. The marine aquarium reference: systems and invertebrates. Plantation, Fla: Green Turtle Publications, 1992.