Recommendations for diagnosing, treating, and preventing heartworm disease
We now have an arsenal of test kits and prophylactics to choose from, and it can be confusing to know which to purchase. We tend to mold ourselves to the product instead of molding the product to the individual patient. This article should help you tailor the heartworm diagnostic, therapeutic, and prophylactic options to each of your canine and feline patients.
Heartworm disease continues to be researched, and new aspects of the disease and its management are discovered every year. We now have an arsenal of test kits and prophylactics to choose from, and it can be confusing to know which to purchase. We tend to mold ourselves to the product instead of molding the product to the individual patient. This article should help you tailor the heartworm diagnostic, therapeutic, and prophylactic options to each of your canine and feline patients.
CANINE HEARTWORM DISEASE
Dogs, coyotes, and wolves are the natural hosts for the spread and development of Dirofilaria immitis. Mosquitoes can transmit infective larvae two to two and a half weeks after ingesting blood from infected dogs.1,2 The development of microfilariae into the infective third-stage larvae (L3) (in the mosquito) requires average daily temperatures greater than 57 F (13.9 C), and transmission is unlikely if the average daily temperature for any 30-day period is less than 65 F (18.3 C).3 So transmission probability varies within the United States depending on the region and ambient seasonal temperature. Dogs housed outside are four to five times more likely to be infected, and thick coats do not reduce the probability of heartworm infections.4 Infective L3 enter a dog's skin through a mosquito bite. After three or four months, the larvae molt into young adults (L5), enter the circulatory system, and eventually travel to the pulmonary arteries.2 Microfilariae (L1) can be seen in the circulatory system six or seven months after the infective L3 enter a susceptible host.
The severity of canine heartworm disease depends on the number of adult heartworms. Dogs with large worm burdens are more likely to develop severe pulmonary hypertension.5 Heartworms in the pulmonary arteries lead to rapid division of the arterial endothelial smooth muscle cell layer and production of collagen (pulmonary arteritis).5 Caudal lobar arteries are predominantly affected (Figure 1).2 This vascular endothelial disease is reversible if the heartworm burden is reduced within four to six weeks of developing an adult heartworm infestation.6
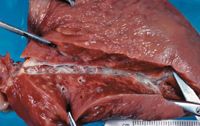
Figure 1: A necropsy specimen of a lung from a dog with heartworm disease. Note the dilated caudal lobar artery with endothelial thickening (arteritis).
Clinical signs and prognosis based on classification
Most dogs with small heartworm burdens are asymptomatic. Coughing secondary to edema (vasculitis) and arteriolar inflammation is the most common clinical sign. Dyspnea associated with parenchymal disease of the caudal lung lobes is also common.2,5 Exercise dramatically increases pulmonary arterial pressure, and exercise intolerance is common with more severe disease.2 If the disease progresses, right-sided congestive heart failure may develop. Physical examination results associated with right-sided congestive heart failure include an abdominal fluid wave (ascites) and decreased lung sounds in the ventral thorax (pleural effusion). Jugular pulses may be present because of increased right atrial pressures. Dyspnea may develop secondary to hypoxemia, and a split second heart sound may occur because of pulmonary arterial hypertension.2
The prognosis for dogs with heartworm disease depends on the severity of the infestation. Class 1 heartworm disease patients have no abnormal physical examination findings or radiographic changes and have an excellent prognosis.
Class 2 patients have mild clinical signs, such as an occasional cough with or without exercise intolerance. Thoracic radiographs show mild pulmonary artery enlargement with mixed alveolar-interstitial lesions (Figure 2). Most Class 2 patients have a good prognosis with proper treatment.
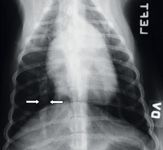
Figure 2: A thoracic radiograph of a dog with heartworm disease. The caudal lobar artery (arrows) is dilated.
Class 3 patients have a poorer prognosis. These patients present with persistent coughing, severe exercise intolerance, weight loss, cachexia, and often right-sided congestive heart failure. Thoracic radiographs show severe pulmonary artery enlargement, right ventricular enlargement, and diffuse pulmonary infiltrates. After heartworm adulticide treatment, recommended adjunctive treatment in Class 3 patients is strict cage rest and oxygen therapy and possibly anti-inflammatory drugs or anticoagulant treatment.7,8
Caval syndrome is present in patients with Class 4 heartworm disease in which the worm burden is so severe (50 to > 200 worms) that the worms drop back into the heart from the pulmonary artery. Worms may extend from the right ventricle across the tricuspid valve and even back into the venae cavae. Patients with caval syndrome present with dyspnea, tachypnea, tachycardia, pale mucous membranes, weakness, and often collapse.9 They are usually anemic and hemoglobinuric because erythrocytes fracture as blood is forced through the mass of worms across the tricuspid valve. Class 4 patients are not candidates for adulticide treatment, and surgical extraction of the heartworms is required (Figure 3).10

Figure 3: Surgical extraction of heartworms through jugular venotomy in a dog with caval syndrome.
Diagnostic tests
Heartworm antigen test kits are widely used in private practices to screen for heartworm infestation. A number of sensitive and specific antigen tests are available. Heartworm antigen tests are based on the presence of mature female worms.11 After successful postadulticide treatment, most antigen test results will be negative by 12 weeks, but occasionally the antigen remains longer (up to five months).12 A study comparing three commercial heartworm antigen test kits found the ELISA Snap RT test (IDEXX Laboratories) had significantly higher sensitivity, accuracy, and negative predictive value than the two other test kits evaluated (VetScan CHAT—Abaxis, Solo Step CH—HESKA).11
Another screening technique is microfilariae testing, though it has largely been replaced by the heartworm antigen test kits. Microfilariae testing is mainly recommended for screening dogs 6 or 7 months of age that have not yet received prophylactic therapy but are ready to start. These patients are screened because heartworm antigens can take up to eight months to appear in the blood after infection. Microfilariae testing must be done in dogs before diethylcarbamazine administration because severe adverse reactions are likely to occur if this drug is given to microfilariae-positive dogs.
Clinicopathologic abnormalities that may be present include eosinophilia, basophilia, nonregenerative or regenerative anemias, hemoglobinuria , thrombocytopenia, inflammatory leukograms, hypoalbuminemia, proteinuria, or disseminated intravascular coagulation indicators.2,13 Thoracic radiography is the most useful diagnostic imaging technique for characterizing the severity of the heartworm disease.14 Echocardiography is useful to evaluate right-sided heart function and to estimate the severity of the pulmonary hypertension and the number and location of the heartworms (Figure 4).
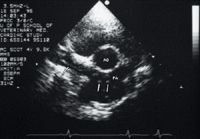
Figure 4: A short-axis right parasternal echocardiogram obtained at the heart base in a dog. Note the linear echo artifacts (arrows) representing the heartworms (PA = pulmonary artery, AO = aorta).
Treatment
Adulticides
The adulticide most frequently administered is melarsomine dihydrochloride. Melarsomine is effective against immature and young adult D. immitis.15,16 Melarsomine is administered by deep intramuscular injections of 2.5 mg/kg in the lumbar epaxial muscles. The recommended therapy for Class 1 and mild-to-moderate Class 2 patients is two injections in alternating epaxial muscle bellies 24 hours apart.17 Certain Class 2 and all Class 3 patients require a modified treatment protocol. A single intramuscular injection is given, followed four to six weeks later with two injections 24 hours apart. About half the heartworms are killed with the initial injection, reducing thromboembolic showering and allowing for the initial pulmonary inflammatory response to subside before the complete worm kill.18 Some researchers advocate treating Class 1, 2, and 3 patients with the split protocol.18
Alternating sides for melarsomine injections is highly recommended. The manufacturer recommends using a 23-ga, 1-in needle for dogs 10 kg or less and a 22-ga, 1.5-in needle for dogs weighing more than 10 kg. Some patients may benefit from sedation for proper deep epaxial intramuscular injections. No more than 4 ml/injection site is recommended. Injection site reactions may arise if the drug migrates out of the injection site along the fascial planes. To help prevent the drug from migrating, use light sedation and limit movement. However, the drug can still migrate and cause ascending inflammation along nerve roots and vasospasm or vasculitis.19 The local inflammation can lead to necrosis and spinal cord compression. Adverse effects of melarsomine include localized swelling, tenderness at the injection site, and possibly mild anorexia. Overdosed patients can be treated with dimercaprol (British antilewisite, or BAL) (3 mg/kg two or three times three hours apart).20 To avoid pulmonary thromboemboli after adulticide therapy, strict rest for three or four weeks is crucial. Exercise restriction one week before adulticide treatment is also recommended.
Microfilaricidal treatment
If circulating microfilariae still exist after adulticidal therapy, microfilaricidal treatment is recommended, but not until four to six weeks after adulticidal therapy. Give milbemycin oxime at a prophylactic dose (0.5 to 1 mg/kg) or ivermectin (50 μg/kg) as a one-time dose, and retest the patient for microfilariae three weeks later. Microfilariae testing is performed rather than immunodiagnostic testing because heartworm antigen can remain for up to three months after elimination of adult heartworms. If microfilariae still exist, repeat the microfilaricidal treatment. If the microfilariae are successfully eliminated, institute prophylactic therapy. Ivermectin at prophylactic doses (6 to 12 μg/kg) is safe to start immediately after adulticidal therapy, and it will slowly eliminate the remaining microfilariae and any remaining adult heartworms.3
Ancillary drug therapy
Ancillary drug therapy is advocated in patients with pulmonary thromboemboli, eosinophilic pneumonitis, eosinophilic pulmonary granulomatosis, or lymphomatoid granulomatosis. Pulmonary thromboemboli are a common complication of heartworm disease and adulticide treatment and usually develop five to 21 days after treatment is initiated. Heparin therapy has been recommended for patients with symptomatic pulmonary thromboemboli subsequent to adulticide treatment or prophylactically to prevent pulmonary thromboemboli in dogs with severe pulmonary arterial disease.7 Heparin (75 to 150 IU/kg subcutaneously t.i.d. with a target activated partial thromboplastin time [aPTT] of one and a half to two times normal) is continued for five to 21 days after the resolution of clinical signs associated with pulmonary thromboemboli and then slowly tapered over several days. In one study, the survival rate was 97.5% in heparin-treated Class 3 dogs compared with 73.5% in Class 3 dogs treated with antiplatelet drugs.7 And postadulticide complications were reduced in heparin-treated patients compared with aspirin-treated patients.7
Corticosteroid therapy remains controversial, but some clinicians still advocate its use for eosinophilic pneumonitis, eosinophilic granulomatosis, and pulmonary infiltrates.8,21 If indicated, prednisone (0.5 to 1 mg/kg orally daily) can be administered until radiographic evidence of the disease shows improvement. Routine prophylactic administration is not recommended as corticosteroids are procoagulant and decrease pulmonary blood flow.8 Oxygen therapy is also recommended if available, as it reduces pulmonary arterial pressures and improves perfusion.22 Oxygen chambers that are temperature-controlled and percent-saturation-controlled are ideal. Nasal insufflation is adequate. The oxygen promotes pulmonary arteriole dilation, decreasing the severity of pulmonary hypertension.
Prophylactic drugs
The choice of prophylactic is often based on a preference for administration frequency (daily [for diethylcarbamazine only] vs. monthly) and whether the dog is at risk of intestinal parasites or ectoparasites. Many excellent prophylactics are available.
The most common prophylactic drugs administered are avermectin- or milbemycin-based. The approved prophylactic ivermectin dose for dogs is 6 to 12 μg/kg orally monthly,23 and selamectin can be applied to the skin monthly (6 mg/kg) for heartworm prophylaxis.24 Moxidectin (ProHeart 6—Fort Dodge Animal Health) is currently not available in an injectable form in the United States. Milbemycin oxime (0.5 to 1 mg/kg) can also be administered orally on a monthly basis for prophylaxis.25
Ivermectin and milbemycin oxime are effective against early L3 infestations. Studies have shown that three-month-old infestations can be greatly reduced with monthly prophylactic doses of either ivermectin or milbemycin oxime, so missing a month or two of prophylaxis is not likely to lead to severe heartworm disease when the patients are receiving these forms of prophylaxis.
Ivermectin is adulticidal at prophylactic doses,26 and monthly administration of ivermectin reduces the adult heartworm burden compared with control dogs.27 The ability of ivermectin to kill young adult worms is called reachback. In one study, initiating milbemycin five and a half months after infecting dogs with 100 infective larvae and continuing milbemycin oxime for one year resulted in no reduction in the worm burden compared with controls (both groups of dogs were necropsied one year after starting therapy). However, initiating ivermectin five and a half months after infestation and continuing ivermectin for one year decreased the worm burden by 56% and decreased it by 35% when ivermectin was started six and a half months after infestation.28 This effect is called trickle kill.
Sixteen continuous months of ivermectin targeted against an adult infection kills 56% of adult heartworms.27 The remaining worms exhibit abnormal motility and appearance. Milbemycin oxime is far less effective against adult infections.27
The American Heartworm Society recommends that adult dogs be evaluated before you initiate prophylaxis and that they be tested annually if they are not receiving year-round preventives.29 Adulticide therapy is the treatment of choice for adult heartworm infestations. The risk for canine heartworm infestation is correlated to signalment, lifestyle, and geography. Prophylaxis is recommended for all dogs at risk of infestation, and dogs should be tested before prophylaxis.29 The trickle kill and reachback capabilities of ivermectin have led to questions regarding the need for annual testing; however, because client compliance is often imperfect, annual testing is recommended. (For comprehensive guidelines on managing canine heartworm infection, see www.heartwormsociety.org.)
FELINE HEARTWORM DISEASE
Although cats are naturally resistant to D. immitis, they are still susceptible to heartworm infestation. The prevalence of heartworm infestation in cats is about 5% to 20% of the rate found in dogs located in the same geographical region.30 Feline heartworm prevalence has been studied in shelter cats naturally exposed to heartworms.31-33 Shelter cats with natural exposure have been necropsied, and in Michigan, the necropsy prevalence is less than 2.5%. In northern Florida, the serologic prevalence is less than 5%. This low prevalence has led to a low index of suspicion and a subsequent low index of diagnosing feline heartworm disease. Feline heartworm disease continues to be a challenge to diagnose. Once cats are infected with L3 larvae, five to eight months will pass before the larvae molt into the L5 stage or young adult worms.34
Clinical signs and diagnostic tests
Clinical signs of feline heartworm disease vary. Dyspnea and coughing are the most common signs, followed by vomiting, collapse, syncope, and sudden death. Murmurs are seldom present unless the cat also has coincidental cardiomyopathy.35 Feline heartworm disease and feline asthma have similar clinical signs. Both conditions can lead to coughing and dyspnea, and both usually respond to corticosteroids, cage rest, and oxygen therapy. Differentiating between the two can be difficult and depends on how aggressively further diagnostic testing is pursued to reach a definitive diagnosis.
Cats usually display clinical signs of heartworm disease when the larvae are in the L4 or L5 stages, making the results of antigen and antibody serology difficult to interpret. To complicate matters even more, antigen and antibody test results can be positive for months after the heartworm infestation has been cleared by the host's natural immune response.36
Antigen testing depends on proteins shed by the parasite. Specifically, antigen tests detect antigen from the sexually mature female reproductive tracts.31 So antigen test results depend on the sex of the worms, the size of the worm burden, and the larval stage (sexually mature females). Male worms, sexually immature worms, or single worm burdens can lead to false negative antigen test results. Positive antigen test results indicate heartworm infestation, but negative test results do not rule out heartworm infestation.35 In a large population of naturally infected cats, antigen tests detected 79% to 86% of the heartworm infections and were highly specific (true negative results).31 So antigen tests are used for confirming infection in antibody-positive cats, and although they are less sensitive, they are more specific.35
Host antibodies develop in response to the early migration of the L3 or L4 larvae and are first detected serologically about two to three months after exposure.34 If an appropriate antibody response occurs, the larvae may be eliminated and may never reach the L5 stage, yet antibodies can persist for several months.35 So positive antibody test results can simply be an indication of exposure without an actual heartworm infestation. Traditionally, antibody tests have been used for heartworm screening because they were reportedly more sensitive (true positive results) than antigen tests,35 but in a recent study, antigen tests outperformed antibody tests when used to identify true positive cases of naturally heartworm-infested cats. Thus, antibody tests should not be used as a stand-alone screening test; rather antigen and antibody tests should be used in combination to increase the sensitivity of heartworm detection.31 Eosinophilia and basophilia are supportive hematologic abnormalities noted in some cats with heartworm infestations.37
Other methodologies for detecting or supporting a diagnosis of a heartworm infestation include thoracic radiography and echocardiography. Caudal pulmonary artery enlargement greater than or equal to 1.6 times the diameter of the ninth rib at the ninth intercostal space is supportive evidence of heartworm infestation.38 Echocardiography is most useful in instances in which antigen test results are negative but cats display clinical and radiographic signs or in cases in which antibody test results are positive and suggest heartworm disease (Figure 5).39
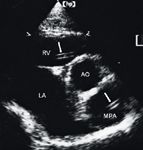
Figure 5: A short-axis right parasternal view echocardiogram showing the aorta (AO), left atrium (LA), right ventricle (RV), and main pulmonary artery (MPA) in a cat. The double echogenic linear structures (arrows) within the right ventricle and main pulmonary artery represent the heartworms.
Prevention and treatment
Feline heartworm prevention is recommended during the transmission period, which is year-round in the southern United States and variable in the northern United States.40 In the Northeast, transmission is from June through November. Infection from D. immitis can be prevented with 2 mg/kg of milbemycin oxime, 0.024 mg/kg of ivermectin administered orally once a month, or 6 mg/kg selamectin applied topically once a month. Prophylaxis can be started at as early as 4 weeks of age. Heartworm-positive cats can receive these preventives without complications, so heartworm testing before administration is not necessary in asymptomatic cats.34
Adulticide therapy with thiacetarsamide sodium (2.2 mg/kg intravenously b.i.d. for two days) is unpredictable and may cause sudden death from embolism of worms. Acute collapse, pulmonary edema, pneumonitis, dyspnea, cyanosis, anorexia, pulmonary thromboemboli, hemoptysis, thrombocytopenia, and sudden death have all been reported from adulticide therapy.34 Therefore, the American Heartworm Society does not recommend arsenical therapy in cats.41 Oxygen therapy, supportive nursing care, and corticosteroids have been advocated in such reactions with variable success. Right heart catheter procedures using various retrieval techniques have successfully removed adult worms from the right atrium, right ventricle, and pulmonary arteries of cats and dogs with caval syndrome. Special care is necessary to avoid tearing worms and causing an acute anaphylaxis reaction. Heartworms in cats have a relatively short life span (two years) compared with heartworms in dogs (five to seven years) because cats are not the normal hosts.34 So in an asymptomatic cat, benign neglect with periodic antigen and antibody monitoring is reasonable. Corticosteroids are often helpful to treat clinical signs resulting from heartworm disease. Preventing an infestation is optimal because it is easier than treatment.41 (For comprehensive guidelines on managing feline heartworm infection, see www.heartwormsociety.org.)
The role of Wolbachia species
Wolbachia species bacteria have an endosymbiotic relationship with D. immitis.42 This rickettsial organism reportedly plays a role in larval embryogenesis, fertility, and maturation of D. immitis.42 The bacteria are found in all the larval stages and are released in considerable amounts during worm molts, microfilariae production, and worm death.43 Researchers have shown that 25 days of tetracycline therapy inhibits Wolbachia species multiplication, thus inhibiting embryogenesis and the maturation of L3 larvae into adult heartworms.42 It has been hypothesized that treating heartworm-positive patients with a 25-day tetracycline course before the adulticide treatment may lessen patients' inflammatory responses to the dying worms. This is strictly hypothetical. Future studies are required to determine the true biology of the gram-negative, rickettsial, endosymbiotic Wolbachia species and its role in heartworm disease.
Kevin J. Christiansen, DVM
Meg M. Sleeper, VMD, DACVIM (cardiology)
Matthew J. Ryan Veterinary Hospital
School of Veterinary Medicine
University of Pennsylvania
Philadelphia, PA 19104-6010
REFERENCES
1. Orihel TC. Morphology of the larval stages of Dirofilaria immitis in dogs. J Parasitol 1961;47:251-262.
2. Rawlings CA, McCall JW, Lewis RE. The response of the canine's heart and lungs to Dirofilaria immitis. J Am Anim Hosp Assoc 1978;14:17-32.
3. Calvert CA, Rawlings CA, McCall JW. Canine heartworm disease. In: Fox PR, Sisson D, Moise SN, eds. Textbook of canine and feline cardiology: principles and clinical practice. Philadelphia, Pa: WB Saunders Co, 1999;702-721.
4. Lewis RE, Losonsky JM. Sex and age distribution of dogs with heartworm disease, in Proceedings. Heartworm Symp 1977;8-9.
5. Rawlings CA, Keith JC, Schaub RG. Development and resolution of pulmonary disease in heartworm infection: Illustrated review. J Am Anim Hosp Assoc 1981;17:711-720.
6. Schaub RG, Rawlings CA. Pulmonary vascular response during phases of canine heartworm disease: scanning electron microscopic study. Am J Vet Res 1980;41:1082-1089.
7. Vezzoni A, Genchi C. Reduction of post-adulticide thromboembolism complications with the low dose heparin therapy, in Proceedings. Heartworm Symp 1989;73-83.
8. Rawlings CA, Keith JC, Schaub RG, et al. Post adulticide treatment pulmonary disease and its modification with prednisolone and aspirin, in Proceedings. Heartworm Symp 1989;122-129.
9. Atkins CE. Pathophysiology of heartworm caval syndrome: recent advances, in Proceedings. Heartworm Symp 1989;27-31.
10. Ishihara K, Sasaki Y, Kitagawa H. Removal of canine heartworms using flexible alligator forceps, in Proceedings 1989;33.
11. Atkins CE. Comparison of results of three commercial heartworm antigen test kits in dogs with low heartworm burdens. J Am Vet Med Assoc 2003;222:1221-1223.
12. Atwell RB, Van Kan DM, Cottis LE, et al. The use of antigen test for diagnosis as an indicator of filarial numbers, and for assessing filarial mortality following thiacetarsamide therapy, in Proceedings. Heartworm Symp 1986;71-76.
13. Rawlings CA, Prestwood AK, Beck BB. Eosinophilia and basophilia in Dirofilaria immitis and Dipetalonema reconditum. J Am Anim Hosp Assoc 1980;16:699-704.
14. Losonsky JM, Thrall DE, Lewis RE. Thoracic radiographic abnormalities in 200 dogs with spontaneous heartworm infections. Vet Radiol 1983;24:120-123.
15. Atwell RB, Searle ACE. Therapeutic efficacy of RM340 in pound dogs infected with Dirofilaria immitis, in Proceedings. Heartworm Symp 1989;143-145.
16. Dzimianski MT, McTier TL, McCall JW. Assessment of filaricidal activity of a new filaricide (RM 340) against immature and adult heartworms using experimental canine nodules, in Proceedings. Heartworm Symp 1989;147-153.
17. Keister DM, Dzimianski MT, McTier TL, et al. Dose selection and confirmation of RM 340, a new filaricide for the treatment of dogs with immature and mature Dirofilaria immitis, in Proceedings. Heartworm Symp 1992;225-229.
18. Atkins CE, Miller MW. Is there a better way to administer heartworm adulticidal therapy? Vet Med 2003;98:310-317.
19. Hettlich BF, Ryan K, Bergman RL, et al. Neurologic complications after melarsomine dihydrochloride treatment for Dirofilaria immitis in three dogs. J Am Vet Med Assoc 2003;223:1456-1461.
20. Atwell RB, Seridan AD, et al. Effective reversal of induced arsenic toxicity using BAL therapy, in Proceedings. Heartworm Symp 1989;155-158.
21. Rawlings CA, Keith JC, Schaub RG. Aspirin and cortico steroids in the treatment of heartworm disease, in Proceedings. Heartworm Symp 1986;139-141.
22. Rawlings CA, Tackett RL. Postadulticide pulmonary hypertension of canine heartworm disease: successful treatment with oxygen and failure of antihistamines. Am J Vet Res 1990;51:1565-1569.
23. Plumb DC. Veterinary drug handbook. 4th ed. Ames, Iowa: Iowa State Press, 2002;454-458.
24. Plumb DC. Veterinary drug handbook. 4th ed. Ames, Iowa: Iowa State Press, 2002;734-735.
25. Blagburn BL, Hendrix CM, Lindsay DS, et al. Postadulticide, milbemycin oxime microfilaricidal activity in dogs naturally infected with Dirofilaria immitis, in Proceedings. Heartworm Symp 1992;159-164.
26. McCall JW, Ryan WG, Roberts RE, et al. Heartworm adulticide activity of prophylactic doses of ivermectin (6 μg/kg) plus pyrantel administered monthly to dogs. In: Seward RL, Knight DH, eds. Recent advances in heartworm disease: symposium '98. Batavia, Ill: American Heartworm Society, 1998:209-215.
27. McCall JW, Guerrero J, Roberts RE. Further evidence of clinical prophylactic, retroactive (reach-back) and adulticidal activity of monthly administrations of ivermectin (Heartgard Plus) in dogs experimentally infected with heartworms, in Proceedings. Heartworm Symp 2002;189-200.
28. Rawlings CA. Effect of monthly heartworm preventatives on dogs with young heartworm infections. J Am Anim Hosp Assoc 2001;38:311-314.
29. American Heartworm Society. 2003 guidelines for the diagnosis, prevention and management of heartworm (Dirofilaria immitis) infection in dogs. Available at: www.heartwormsociety.org/AHS%20Guidelines-Canine.htm. Accessed May 2005.
30. Ryan WG, Newcomb KM. Prevalence of feline heartworm disease—a global review, in Proceedings. 8th Heartworm Symp 1995;79-86.
31. Berdoulay P, Levy JK, Snyder PS, et al. Comparison of serological tests for the detection of natural heartworm infection in cats. J Am Anim Hosp Assoc 2004;40:376-384.
32. Kalkstein TS, Kaiser L, Kaneene JB. Prevalence of heartworm infection in healthy cats in the lower peninsula of Michigan. J Am Vet Med Assoc 2000;217:857-861.
33. Hermesmeyer M, Limberg-Child RK, Murphy AJ, et al. Prevalence of Dirofilaria immitis infection among shelter cats. J Am Vet Med Assoc 2000;217:211-212.
34. Dillon R. Feline heartworm disease. In: Fox PR, Sisson D, and Moise SN, eds. Textbook of canine and feline cardiology: principles and clinical practice. Philadelphia, Pa: WB Saunders Co, 1999;727-734.
35. Dillon R, Brawner WR, Robertson-Plouch CK, et al. Feline heartworm disease: correlations, clinical signs, serology, and other diagnostics—results of a multicenter study, in Proceedings. Heartworm Symp 1998;153-157.
36. Knight DH, Downing R, Nelson CT, et al. 2002 guidelines for diagnosis, prevention, and management of heartworm (Dirofilaria immitis) infection in cats. Am Heartworm Soc Bull 2002;29:2-11.
37. Atkins CE, DeFrancesco TC, Coats JR, et al. Heartworm infection in cats: 50 cases (1985-1997). J Am Vet Med Assoc 2000;217:355-358.
38. Schafer M, Berry CR. Cardiac and pulmonary artery mensuration in feline heartworm disease. Vet Radiol Ultrasound 1995;35:499-505.
39. DeFrancesco TC, Atkins CE, Miller MW, et al. Use of echocardiography for the diagnosis of heartworm disease in cats: 43 cases (1985-1997). J Am Vet Med Assoc 2001;218:66-69.
40. Levy JK, Snyder PS, Taveres LM, et al. Prevalence and risk factors for heartworm infection in cats from northern Florida. J Am Anim Hosp Assoc 2003;39:533-537.
41. 2002 guidelines for the diagnosis, prevention, and management of heartworm (Dirofilaria immitis) infection in cats. Batavia, Ill: American Heartworm Society, 2002;1-8.
42. Bandi C, McCall JW, Genchi C, et al. Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. Int J Parasitol 1999;29:357-364.
43. Morchon R, Ferreira AC, Martin-Pacho JR, et al. Specific IgG antibody response against antigens of Dirofilaria immitis and its Wolbachia endosymbiont bacterium in cats with natural and experimental infections. Vet Parasitol 2004;125:313-321.