Severe pulmonary hypertension and cardiovascular sequelae in dogs
Pulmonary hypertension is increased blood pressure in the pulmonary vascular system.
PULMONARY HYPERTENSION is increased blood pressure in the pulmonary vascular system. In the past, the most common cause of pulmonary hypertension in dogs was heartworm infestation. The mechanical and immunologic effects of the worms can lead to various degrees of increased pulmonary vascular resistance and pressure. The widespread use of heartworm preventives has presumably reduced the frequency of pulmonary hypertension. However, nonheartworm-related pulmonary hypertension is also a clinical problem. Practitioners can now more easily distinguish between heartworm-related and nonheartworm-related cases by knowing a patient's heartworm prevention history and the results of heartworm antigen testing and echocardiographic examination.
Regardless of the cause, echocardiography can often noninvasively estimate intravascular and intracavitary pressures as well as identify morphologic cardiac changes in dogs with various degrees of pulmonary hypertension.1 This ability to quickly and easily identify cases of pulmonary hypertension has been an important diagnostic advance and has greatly reduced the need for cardiac catheterization.
Canine pulmonary hypertension ranges widely in severity. Pulmonary hypertension associated with left heart failure is usually mild, whereas dogs with advanced pulmonary disease can have peak systolic pressures in the right ventricle and pulmonary artery that are four to five times normal (normal = 20 to 25 mm Hg). In our laboratory, the estimated pressures are categorized as mild (30 to 55 mm Hg), moderate (56 to 79 mm Hg), and severe (> 79 mm Hg).
This article presents two cases of older dogs with severe pulmonary hypertension. These dogs had peak right ventricular systolic pressures in excess of 79 mm Hg, and their cases highlight three important aspects of pulmonary hypertension:
1. The difficulty in understanding the primary cause and pathophysiology of this life-threatening condition.
2. The cardiac manifestations of severely elevated pulmonary vascular pressure (cor pulmonale).
3. The challenge of planning and implementing a therapeutic protocol.
Case 1
A 14-year-old, 36.4-lb (16.5-kg), mixed-breed, spayed female dog was presented to the referring veterinarian with a history of acute dyspnea, coughing, and anorexia. Auscultation of the lung fields had revealed diffuse crackles. The results of a heartworm antigen test done eight months earlier were negative, and the dog had been receiving a monthly heartworm preventive. The veterinarian had suspected that the dog had congestive heart failure and had instituted treatment with furosemide, enalapril, and oxygen before referral to the Veterinary Teaching Hospital at Virginia Tech.
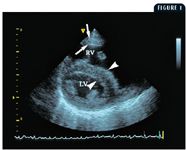
1. This right short-axis echocardiogram demonstrates the thickened right ventricular free wall (arrows), the large right ventricle (RV), and the flattened interventricular septum (arrowheads) of the dog in Case 1. The displacement of the septum to the left and the diminished blood flow through the pulmonary vascular system can combine to cause the left ventricle (LV) to be smaller than normal.
Initial examination and diagnostic tests
The dog was presented to Virginia Tech five days after the examination by the referring veterinarian. The initial examination revealed weakness, stress-induced cyanosis, and labored breathing. The dog's rectal temperature was 101.3 F (38.5 C), its heart rate was 140 beats/min, and its respiration rate was 70 breaths/min. A grade II/VI systolic murmur was heard over the tricuspid valve area. The lung sounds were harsh and at times were suggestive of a pleural friction rub. An electrocardiographic examination indicated mild right axis deviation. Thoracic radiographs showed right heart enlargement, main pulmonary artery enlargement, and a mild, diffuse interstitial pattern. Repeated pulse oximetry readings averaged 55%. No abnormalities were seen on abdominal ultrasonographic examination. A complete blood count revealed a stress leukogram; the total white blood cell count was 25.8 × 103 /µl (normal = 4.6 to 17.8 × 103 /µl), with 22.962 × 103 /µl segmented neutrophils (normal = 2.8 to 12.8 × 103 /µl), 0.774 × 103 /µl band neutrophils (normal = 0), 0.516 × 103 /µl lymphocytes (normal = 1 to 3.7 × 103 /µl), and 1.548 × 103 /µl monocytes (normal = 0.09 to 0.9 × 103 /µl). Abnormal serum chemistry profile results were elevated blood urea nitrogen (57 mg/dl; normal = 8 to 27 mg/dl) and creatinine (1.8 mg/dl, normal = 0.6 to 1.4 mg/dl) concentrations. The dog's urine specific gravity was 1.008.
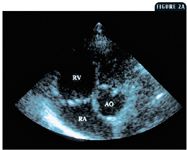
2A. A left caudal parasternal long-axis view showing the enlarged right ventricle (RV) and right atrium (RA) of the dog in Case 1 (AO = aorta).
Echocardiographic examination
An echocardiographic examination revealed a right ventricle that was grossly dilated and appeared larger than the left ventricle. The right ventricular free wall was thick (0.6 cm) compared with the left ventricular free wall (0.9 cm), suggesting that this dog had right ventricular hypertrophy. The increased pressure or volume in the right ventricle displaced the interventricular septum to the left, making the septum appear flattened. The displacement of the septum as well as the potentially decreased blood flow to the left side of the heart due to reduced pulmonary blood flow resulted in a left ventricle that was smaller than expected. The M-mode values from the right short-axis view showed a left ventricular diastolic diameter of 2.14 cm (normal = 3.4 cm) and left ventricular systolic diameter of 1.43 cm (normal = 2.1 cm) (Figure 1). Substantial tricuspid regurgitation could be seen from several imaging planes (Figures 2A & 2B).
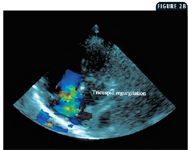
2B. The same view as in Figure 2A but with color Doppler applied to the two-dimensional image. The green reflects the substantial tricuspid regurgitation during ventricular systole. This view allows for good alignment between the regurgitant jet and the Doppler beam, providing an accurate assessment of jet velocity.
Continuous wave Doppler echocardiography was used to measure the velocity of the tricuspid regurgitant jet from the left caudal parasternal window (Figure 2B). This view allowed excellent Doppler alignment (± 20 degrees) with the jet so that the flow profile was an accurate representation of the blood velocity between the right ventricle and the right atrium. The velocity of the jet consistently ranged between 4.5 and 5 m/s (Figure 2C). Using the modified Bernoulli equation (P = 4V2 ) to convert the peak velocity of the regurgitant jet to a pressure (P = pressure gradient in mm Hg; V2 = peak velocity distal to an orifice or obstruction), we estimated that the range was 81 to 100 mm Hg (normal = 20 to 25 mm Hg) before adding an estimated right atrial pressure (6 mm Hg). So the peak systolic pressure in the right ventricle and pulmonary arterial system ranged from 87 to 106 mm Hg. We eliminated pulmonic stenosis as a cause of the right ventricular hypertension by evaluating the right ventricular outflow tract and pulmonary valve region. A pulmonary regurgitant jet could not be identified, so it was not possible to estimate the diastolic pressure in the pulmonary artery.
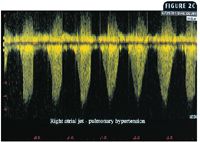
2C. This continuous wave Doppler profile was made from an image similar to that displayed in Figure 2B. The scale to the left reveals that the peak right atrial jet velocity was between 4.5 and 5 m/s. With the modified Bernoulli equation, this translates to a systolic pressure gradient of 81 to 100 mm Hg (normal = 20 to 25 mm Hg) and suggests that the peak systolic right ventricular and pulmonary artery pressure was at least 81 mm Hg.
Treatment and outcome
While it was appreciated that pulmonary parenchymal disease could be the underlying problem in this dog, the immediate threat was the severe pulmonary hypertension. Thus, the therapeutic plan included steps to reduce pulmonary vascular resistance and pressure. Therapy included 40% cage oxygen, sildenafil citrate (we decided to administer a dosage of 1.5 mg/kg orally b.i.d. and gradually increased it over five days to 6 mg/kg every four to six hours), and heparin (100 U/kg subcutaneously q.i.d.).
Repeated echocardiographic studies were done to assess the effectiveness of the therapy. While some peak right ventricular systolic pressure measurements were as low as 50 mm Hg, the pressure never consistently remained reduced, and the dog did not improve clinically. After five days of continuous oxygen therapy, increasing doses of sildenafil (maximum dose of 6 mg/kg orally four to six times a day), heparin, amoxicillin (24 mg/kg b.i.d., added on Day 3), and furosemide (3 mg/kg t.i.d., added on Day 4), the dog was euthanized.
A complete necropsy was performed. The lungs were mottled and rubbery, and the lung peripheries were firmer than the hilar portions. No thrombi were present in the pulmonary arteries. The heart was dilated with a hemorrhagic right auricle. The kidneys were grossly normal. Histologically, the kidneys showed scattered interstitial areas that were infiltrated with lymphoid cells. Pulmonary alveoli were filled with neutrophils and macrophages, and some contained fibrin. Many of the smaller pulmonary arteries were thickened because of smooth muscle cell proliferation (Figure 3). The right ventricular wall had scattered foci of interstitial fibrosis and degenerate myocytes. Cultures of the pulmonary parenchyma were not done. The pathologic diagnoses were cardiomyopathy and suppurative interstitial pneumonia.
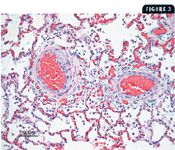
3. A photomicrograph of a specimen from the lung of the dog in Case 1. The two pulmonary arteries in this section demonstrate advanced arteriosclerosis. There is marked thickening of the tunica media and tunica adventitia. The thickening of the tunica media is due to proliferation of muscle fibers, and there is increased fibrous tissue in the tunica adventitia. The vascular thickening is presumably an important factor in the development of pulmonary hypertension (hematoxylin-eosin).
Case 2
A 13-year-old, 68-lb (30.9-kg), spayed female Labrador retriever was presented to the referring veterinarian with a history of gagging, retching, coughing, and respiratory stridor. The dog had been receiving a heartworm preventive, and the most recent antigen test result (obtained 22 months before presentation) had been negative. A thoracic radiographic examination had revealed only small, diffuse pulmonary calcifications. The referring veterinarian had concluded that the dog had chronic bronchitis and laryngeal paralysis. Treatment included enrofloxacin and aminophylline. The dog improved initially but had returned three months later with worsening respiratory signs. An electrocardiographic examination had showed occasional right-ventricular-originating premature beats. Laboratory testing had revealed a low serum thyroxine concentration, so levothyroxine sodium had been added to the therapeutic plan.
Seven months after the initial presentation for the respiratory problem, arytenoid lateralization was performed to alleviate the respiratory obstruction. For 10 days after the surgery, the dog had profound weakness, respiratory stridor, cardiac arrhythmia, and one syncopal episode. Three weeks after the surgery, the dog was referred to the Veterinary Teaching Hospital at Virginia Tech for evaluation of the cardiac arrhythmia. At the time of referral, the dog was being treated orally with levothyroxine (0.016 mg/kg b.i.d.), procainamide (16 mg/kg t.i.d.), theophylline (9.7 mg/kg b.i.d.), dexamethasone (0.008 mg/kg every 48 hours), and carprofen (2.4 mg/kg b.i.d.).
Initial examination, diagnostic tests, and treatment
On physical examination, the dog's rectal temperature was 101.6 F (38.7 C), its pulse rate was 180 beats/min, and its pulse quality was weak. The dog was panting heavily. No heart murmurs or abnormal pulmonary sounds were auscultated. The results of an echocardiographic examination were normal. An electrocardiographic examination revealed occasional right ventricular premature beats. The cardiac silhouette was normal on thoracic radiographs, but there was a mild, diffuse interstitial lung pattern that was considered a secondary complication to respiratory compromise. The radiographs also showed numerous radiopaque, small nodules primarily in the cranial lung lobes that were thought to be age-related calcification. The results of a complete blood count were normal.
Since the clinical signs in this dog were not entirely attributable to the cardiovascular system, a consult from the surgery service was requested to evaluate the laryngeal paralysis and the prior surgery. The dog was anesthetized for a brief period, and visual inspection of the laryngeal area revealed bilateral obstruction and substantial laryngeal and perilaryngeal swelling. The surgical procedure had not successfully reduced the laryngeal obstruction, so a right arytenoid lateralization was performed within 48 hours of presentation to the teaching hospital.
Further diagnostic tests and treatment
The dog continued to cough, had limited exercise tolerance, and was weak in the first few days after surgery. Continuous nasal oxygen insufflation was provided. Other therapies included carprofen (2.4 mg/kg b.i.d.), levothyroxine (0.016 mg/kg b.i.d.), theophylline (9.7 mg/kg t.i.d.), amoxicillin (23 mg/kg t.i.d.), and intravenous fluids. Several cardiovascular evaluations were done during the postoperative period.
Five days after the surgery, physical examination revealed increased frequency of coughing; loud, bilateral pulmonary crackles; and one episode of collapse. Three blood gas analyses were done during this period, and the partial pressure of oxygen (PO2) in arterial blood averaged 58 mm Hg (normal = 84.7 to 116.1 mm Hg). An electrocardiographic examination revealed frequent ventricular premature beats with an occasional three-consecutive-beat pattern (intermittent ventricular tachycardia). Thoracic radiographs showed right ventricular enlargement and an enlarged pulmonary artery (Figures 4A & 4B).
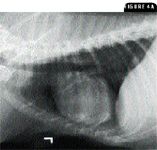
4A. This lateral thoracic radiograph of the dog in Case 2 shows moderate cardiomegaly. The trachea is elevated. There are multiple small radiopaque lesions most visible over the heart shadow. These small opacities were considered to be age-related and not necessarily linked to pulmonary hypertension.
Echocardiographic findings were dramatically changed from the original examination five days earlier. The right ventricle was enlarged, and the interventricular septum was flattened. The left ventricular diameter from the right short-axis view was markedly diminished. The right short-axis M-mode diastolic diameter was 2.58 cm (normal = 4.3 cm), and the systolic diameter was 0.7 cm (normal = 2.8 cm). There was substantial tricuspid and pulmonary valve regurgitation. The right ventricular free wall was thicker (1.48 cm) than the left ventricular free wall (1.41 cm).
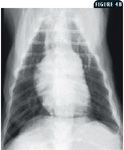
4B. This dorsoventral thoracic radiograph presents a classic reversed D sign due to right ventricular hypertrophy. At the 2-o'clock location, there is an enlarged pulmonary artery segment.
Using the tricuspid jet peak velocity measurement, spectral Doppler echocardiography, and the modified Bernoulli equation, we determined that the peak systolic right ventricular and pulmonary artery pressures were about 100 mm Hg. Using the end-diastolic velocity, spectral Doppler echocardiography, and the modified Bernoulli equation, we determined the end-diastolic pulmonary artery pressure was at least 20 mm Hg (normal = 10 mm Hg). In an effort to reduce pulmonary vascular resistance and pressure, treatment with an α-adrenergic blocking agent (2 mg prazosin hydrochloride orally t.i.d.) was begun.2 In two subsequent echocardiographic examinations done six and eight days postoperatively, the right ventricular and pulmonary artery pressures remained severely increased. Additional therapy included prednisone (0.5 mg/kg orally once a day) and enrofloxacin (5 mg/kg orally b.i.d.). The prednisone was given to reduce inflammatory changes and swelling in the upper and lower respiratory tract in an effort to improve gas exchange. The enrofloxacin was used because of a concern that the dog might aspirate gastric contents. Blood gas analysis continued to reveal low PO2 levels.
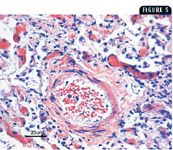
5. A photomicrograph of a specimen from the lung of the dog in Case 2. There is marked tunica adventitia thickening and mild tunica media thickening in the pulmonary artery. In addition, there is marked inflammatory reaction compatible with infectious or inflammatory pulmonary disease (hematoxylin-eosin).
Nine days after surgery, the dog was discharged from the hospital. The owner was told that the prognosis was poor because of severe pulmonary hypertension and general unresponsiveness to therapy. The therapeutic plan at discharge included enrofloxacin (5 mg/kg orally b.i.d.), amoxicillin (23 mg/kg orally t.i.d.), prazosin (2 mg orally t.i.d.), and prednisone (0.25 mg/kg orally b.i.d.). The carprofen and levothyroxine were discontinued.
Follow-up and outcome
The dog was returned to the teaching hospital a week after discharge. The owner indicated that coughing and general weakness continued. An arterial blood gas analysis (pH = 7.365, normal = 7.324 to 7.459; PCO2 = 24.9 mm Hg, normal = 27.7 to 41.2 mm Hg; PO2 = 64.9 mm Hg, normal = 84.7 to 116.1 mm Hg; oxygen saturation = 91.3%, normal = 96% to 98.2%) continued to reveal severe hypoxemia. The prednisone was discontinued but the antibiotics and prazosin were continued.
Four additional teaching hospital visits were made over the ensuing 10 weeks. Although the dog was never without clinical signs related to hypoxemia, there was a period when the echocardiographic signs of cor pulmonale improved. While substantial right ventricular hypertrophy remained, the right ventricular chamber appeared to be smaller than at previous examinations, and the tricuspid regurgitation was minimal in volume and at times was difficult to identify. During this time, the pulmonary regurgitation jet could not be seen, but the PO2 in the arterial blood remained at 60 mm Hg. Several complete blood counts were performed in this dog, and they did not show evidence of polycythemia. Therapy included 20 mg/kg theophylline given orally twice a day and 0.33 mg/kg prednisone given orally every other day.
At an evaluation done 12 weeks after the surgery at the teaching hospital, the dog was exhibiting extreme dyspnea, and the owner reported a recent syncopal episode and seizure. Since the dog's quality of life was unacceptable to the owner, the dog was euthanized. A complete necropsy was performed.
The necropsy revealed an enlarged spleen with tan foci throughout. Histologic examination of the spleen showed multiple lipid foci with some hematopoietic cells. The right medial and lateral liver lobes were enlarged and adhered by fibrin. Multiple tan nodules were noted in all liver lobes. Histologic evaluation of the liver revealed portal fibrosis. Grossly, there were numerous 1- to 2-mm mineralized masses in the pleura and all lung lobes. The caudal lung lobes had distinct red areas that formed a mosaic pattern. Histologically, the terminal bronchioles contained lymphocytes, plasma cells, and neutrophils, and these cells extended into the adjacent alveoli. Cultures of the pulmonary parenchyma were not done. Arteriosclerosis was evident in some pulmonary arteries (Figure 5), and in situ thrombosis was apparent. Right ventricular hypertrophy was noted. The pathologic diagnoses were splenic lipomas with hematopoiesis, muscular atrophy of the larynx, branchial cyst of the thymus, mild bronchopneumonia, and adnexal dysplasia of the skin.
Types of pulmonary hypertension
Pulmonary hypertension is either primary or secondary. Primary pulmonary hypertension cases are those without a known cause; secondary pulmonary hypertension cases relate to discernible causes.3 Primary pulmonary hypertension in dogs is rare.4 Secondary pulmonary hypertension in people is a common sequela of chronic obstructive pulmonary disease (COPD). A diagnosis of COPD in people is based on abnormal expiratory flow results that do not change spontaneously over short periods or after administration of bronchodilators.5 The two most common causes of COPD are bronchitis and emphysema. Secondary pulmonary hypertension in dogs can be caused by many factors, including bronchiectasis, emphysema, infiltrative pulmonary diseases, pulmonary thromboembolism, and heartworms.1
Control of pulmonary vascular tone
In recent years, great progress has been made in understanding the mechanisms involved in the control of pulmonary arterial function. Multispecies research has shown that pulmonary vascular resistance can vary widely, and, increasingly, the endothelial cells lining the pulmonary vessels appear to play a principal role because they are directly or indirectly involved with many of the substances that can cause vasodilation or vasoconstriction of the pulmonary arteries.3 Indeed, endothelial cells can look normal histologically but function abnormally. Prostaglandins, produced by lung tissue, are important in pulmonary vascular regulation. PGI2 (prostacyclin) and PGE2 are vasodilators, while PGF2α and PGA2 are vasoconstrictors. Prostacyclin is also released by the endothelial cells. In addition to causing vasodilation, it can inhibit platelet aggregation by activating adenylate cyclase. Nitric oxide has a biologic action similar to prostacyclin in that it causes relaxation of vascular smooth muscle. This is the rationale for using sildenafil in patients with pulmonary hypertension. Nitric oxide is released from endothelial cells in response to physiologic stimuli including thrombin, bradykinin, and blood flow (shear stress). Nitric oxide inhibits platelet activation and creates an antithrombotic property on the endothelial surface.3
Vasoconstrictors include thromboxane from platelets and macrophages, endothelin from endothelial cells, and angiotensin II that is generated in the lung from the conversion of angiotensin I. Endothelin has a long half-life, which can lead to prolonged vasoconstriction. Serotonin, derived from platelets, can be a vasodilator or vasoconstrictor depending on the clinical circumstances. Serotonin can act as a growth factor and contribute to vascular medial hypertrophy and promote vascular remodeling.3
Low alveolar oxygen tension is a strong stimulus for rapid pulmonary vasoconstriction. This vasoconstriction is a well-recognized adaptive mechanism for shunting blood flow away from poorly ventilated areas of the lung to better ventilated areas. Hypoxia inhibits outward potassium currents throughout the pulmonary vasculature, resulting in depolarization of the pulmonary vascular smooth muscle. This depolarization allows calcium entry into voltage-dependent calcium channels, promoting vascular contraction. Calcium can also be mobilized intracellularly from the sarcoplasmic reticulum, mitochondrial membrane, and inner aspect of the cell membrane. A reduction in nitric oxide production has been demonstrated in chronically hypoxic piglets and rats, and prolonged inhalation of nitric oxide attenuates hypoxic pulmonary vasoconstriction and vascular remodeling.3,5
Changes in alveolar oxygenation can directly affect the oxygenation of small pulmonary arteries and arterioles by direct gaseous diffusion as well as promote low oxygen tension in the blood of these small vessels. The arteries and arterioles appear to be the primary site for vasoconstriction and increased resistance during hypoxia. Acidosis frequently accompanies hypoxia and can act synergistically with hypoxia to promote pulmonary vasoconstriction.5
Vascular remodeling from hypoxia is mediated by growth factors including platelet-derived growth factor A and B in hypoxic rats.5 Vascular endothelial growth factor is an endothelial-cell–specific mitogen that can be involved in pulmonary vascular injury and endothelial cell proliferation because of its permeability, angiogenesis, proinflammatory properties, and specificity for endothelial cells. Angiotensin-converting enzyme (ACE) and angiotensin II may play important roles in the development of right ventricular hypertrophy. ACE inhibitors have been shown to attenuate the development of pulmonary hypertension in rats exposed to chronic hypoxia.5
Cardiac effects of pulmonary hypertension
The right ventricle is particularly affected by pulmonary hypertension. A normal right ventricle has an output that is equivalent to that of the left ventricle; however, normal pulmonary artery pressure is much lower than the systemic blood pressure, making the design and function of the two ventricles quite different.6 The right ventricular free wall is thin, making it a highly compliant chamber that can easily accommodate increases in filling pressures. Systolic pressures in the right ventricle and pulmonary artery are usually less than 30 mm Hg, and the right ventricular end-diastolic pressure is usually less than 6 mm Hg. Mean pulmonary artery pressures range from 10 to 18 mm Hg. The thin-walled right ventricle is not suited to the development of high systolic pressures, and an acute increase in outflow impedance is poorly tolerated. If the pulmonary hypertension develops gradually, the right ventricle is better able to adapt to the increase in workload. In chronic cases, the right ventricle hypertrophies and becomes less compliant, and wall tension and contractility increase. These structural and functional cardiac changes secondary to respiratory disease are commonly referred to as cor pulmonale.6 In people, chronic cor pulmonale implies obstructive or restrictive lung disease, while acute cor pulmonale suggests acute pulmonary hypertension due to massive pulmonary embolism.5
The echocardiographic manifestations of pulmonary hypertension can be dramatic, and, generally, the more severe the pulmonary hypertension, the more apparent the changes. Dogs with advanced respiratory disease and hyperinflation of the lungs can be difficult to image because of imaging window reduction. The advanced cases usually demonstrate moderate to severe right ventricular free wall hypertrophy and right ventricular dilatation. Interventricular septal flattening is often easily recognized. Severe flattening is usually identified in cases in which the right ventricular pressures approximate or exceed left ventricular systolic pressures. Elevated right ventricular diastolic pressures can cause paradoxical interventricular septal motion in which the septum moves to the left during diastole as a result of increased right ventricular pressure and volume overload. The main pulmonary artery may appear enlarged and, if so, pulmonary regurgitation is often present. It is important to interrogate the right ventricular outflow tract and pulmonary valve to eliminate pulmonary stenosis as a cause of right ventricular hypertension; however, most dogs with pulmonary hypertension are middle-aged or older, which reduces the possibility of an unrecognized congenital lesion. The left ventricle may appear small when compared with the large right ventricle or may actually be small because of low cardiac output secondary to pulmonary vascular obstruction. Furosemide administration can also reduce the size of the left ventricle. With substantial tricuspid regurgitation, the right atrium may be enlarged.1,4,7
Spectral Doppler echocardiography provides an effective, noninvasive technique for estimating right ventricular or pulmonary artery pressures. For pulmonary artery and right ventricular systolic pressures, it is important to locate a tricuspid valve regurgitant jet. These jets are usually easy to identify, particularly in severe cases. While the jet can usually be found from the right parasternal view, the best windows are the left caudal parasternal and left cranial parasternal. The peak jet velocity and the modified Bernoulli equation are used to calculate the systolic pressure in the right ventricle and pulmonary artery. This gradient is added to the estimated pressure in the right atrium to provide the estimated peak pressures. In a dog not in heart failure, the right atrial pressure is estimated at 5 to 6 mm Hg. With right heart failure, the right atrial pressure is estimated to fall between 10 and 15 mm Hg. In a similar way, the peak velocity of a pulmonary artery regurgitant jet and the modified Bernoulli equation are used to estimate pulmonary artery diastolic pressure. The best view for identifying a jet of pulmonary regurgitation is the right parasternal short-axis view. The jet can also be identified from the left cranial parasternal window. In my experience, the jet of tricuspid regurgitation is a more common finding than the jet of pulmonary regurgitation in dogs with moderate to severe pulmonary hypertension.
Treating pulmonary hypertension
Therapy for primary pulmonary hypertension in people can include oxygen, digoxin, adenosine, prostacyclin infusion, nitric oxide, and high-dose calcium channel blockers.3 Treatment of secondary pulmonary hypertension in people may involve oxygen, anticholinergics, β-adrenergic agonists, theophylline, corticosteroids, digitalis, nitric oxide, and ACE inhibitors. In people with COPD, only oxygen has been shown to produce consistent pulmonary vasodilation.5 Oxygen relieves pulmonary vasoconstriction, which allows right ventricular stroke volume to increase, enhancing oxygen delivery to the vital organs. Sildenafil has been shown to reduce hypoxia-induced pulmonary hypertension in people and mice,8,9 but its use in dogs has been limited.
Recommended treatment of pulmonary hypertension and associated problems in dogs includes oxygen, antibiotics, bronchodilators, calcium channel blockers, α-adrenergic blocking agents, salt restriction, ACE inhibitors, and diuretics.4,10 The primary therapy in the two dogs in this report was supplemental oxygen. In both dogs, clinical and echocardiographic improvement in the manifestation of pulmonary hypertension was not documented except for a possible transient improvement in the echocardiographic findings in the Labrador retriever (Case 2). During this period, the animal was not receiving supplemental oxygen. In ambulatory people, long-term oxygen administration is much easier to accomplish than in dogs and has been documented to be helpful. People with COPD often need oxygen supplementation 12 or more hours a day.5 The heparin used in the 14-year-old mixed breed dog (Case 1) was directed at preventing and resolving any potential thromboembolic aspects of this animal's problem.
Review of the presented cases
The two cases presented in this article are part of a group of nearly 54 dogs with various degrees of pulmonary hypertension (15 were severe) studied over two and a half years at the Virginia-Maryland Regional College of Veterinary Medicine. The prognosis and cardiac changes in the 54 dogs parallel the underlying severity of the pulmonary hypertension problem. The presented cases represent the severe form of pulmonary hypertension and reveal the difficulty in identifying the problem before it becomes irreversible. In addition, there is the challenge of understanding the pathophysiology in each case. Control of pulmonary vascular resistance and pressure is a finely tuned and complex system that can become imbalanced in many ways. To appropriately treat animals with the more severe forms of pulmonary hypertension, comprehensive physiologic monitoring could be of great benefit; however, the invasive procedures needed to monitor intracardiac pressures, cardiac output, and vascular resistance may be difficult to accomplish in critically ill patients.
At necropsy, the two dogs in this study had no evidence of heartworm disease or major pulmonary embolism. There was evidence of in situ thrombosis in the Labrador retriever.
Presumably, the pulmonary vascular hypertension in these dogs was related secondarily to pulmonary parenchymal disease. Both dogs had pathologic evidence of inflammatory pulmonary disease; however, the chronicity is open to question, particularly in the 14-year-old mixed-breed dog. This dog presented with an acute history of dyspnea and coughing and was referred quickly to Virginia Tech. The Labrador retriever had a much more chronic history and was referred many months after initial presentation to the referring veterinarian. The severity of the laryngeal paralysis and accompanying hypoxia could have played an important role in this animal's clinical course and development of pulmonary hypertension. Clearly, both dogs had documented hypoxemia, and it is well-known that this can promote pulmonary vasoconstriction; however, beyond the direct effect of hypoxia-induced vasoconstriction through potassium and calcium availability to the vascular smooth muscle cells, it is not known what other vascular control factors might have been disturbed. Disequilibrium of the vasoconstrictors and vasodilators that were discussed above could have played a principal role, but assessing this is not easily done in the clinical setting.
It is tempting to speculate on what effect abnormal pulmonary vascular function might have on other vessels. The systemic vessels, including the coronary arteries, are controlled in a manner similar to the pulmonary vessels. Perhaps the disequilibrium in the pulmonary vessels might liberate substances or trigger mechanisms that could influence systemic vessels. For example, endothelin is a potent vasoconstrictor with a long half-life and is important in pulmonary vascular control. Increased production of this circulating substance could cause constriction of systemic vessels, including the coronary arteries. Coronary vasoconstriction concurrent with a stressed right ventricle in a dog with severe pulmonary hypertension could be devastating. The frequent and long-standing premature ventricular contractions seen in the Labrador retriever could have been the result of reduced coronary blood flow associated with a stressed right ventricle trying to deal with an enormously increased afterload.
Finally, these two cases illustrate the challenge of trying to successfully treat the severe form of pulmonary hypertension. By examining the original group of 15 severe cases (including the two in this report), it is apparent that such dogs have a grave prognosis, irrespective of the therapeutic plan, and usually survive a few days to a few weeks.
Future considerations
Dealing with severe pulmonary hypertension in dogs is rewarding from the recognition perspective. The technology behind echocardiography, including transesophageal studies, is constantly improving, and it will become easier to recognize the vascular and cardiac manifestations of pulmonary hypertension. The frustrating aspect of this problem is to understand the pathogenesis and pathophysiology. While we assume that pulmonary parenchymal disease precedes pulmonary vascular disease, this is not always obvious. Some dogs have no physical or radiographic signs of pulmonary parenchymal disease but have severe pulmonary hypertension. The lack of necropsy studies in many cases makes final judgments about parenchymal disease difficult at best.
Developing a rational therapeutic plan is essential in these cases, and providing oxygen is clearly a reasonable step. However, the complex cascade of events involved in controlling pulmonary vascular tone could easily be disturbed in a number of ways. In the clinical setting, there is no practical way to evaluate the pulmonary vascular tone or assay the substances that play a principal role in controlling pulmonary vascular tone, so each case becomes an experiment in finding a plan that will substantially reverse the pulmonary hypertension.
A better understanding of nonheartworm pulmonary hypertension in dogs will result from appropriate research. Further characterization of parenchymal diseases of the lung as well as a comprehensive evaluation of the pulmonary vascular abnormalities will be critical. Assays of control substances and histochemical studies of the pulmonary vessels may be particularly rewarding. Progress is being made through multispecies studies, and this is likely to continue. In time, the treatment and management of canine pulmonary hypertension may be less empirical and more rational.
R. Lee Pyle, VMD, MS, DACVIM (cardiology)
Jonathan Abbott, DVM, DACVIM (cardiology)
Heidi MacLean, DVM
Department of Small Animal Clinical Sciences
Virginia-Maryland Regional College of Veterinary Medicine
Virginia Tech
Blacksburg, VA 24061
REFERENCES
1. Johnson, L. et al.: Clinical characteristics of 53 dogs with Doppler-derived evidence of pulmonary hypertension: 1992-1996. J. Vet. Intern. Med. 13 (5):440-447; 1999.
2. Salvi, S.S: Alpha1-adrenergic hypothesis for pulmonary hypertension. Chest115 (6):1708-1719; 1999.
3. Rich, S.: Pulmonary hypertension. Heart Disease: A Textbook ofCardiovascular Medicine (E. Braunwald et al., eds.). W.B. Saunders, Philadelphia, Pa., 2001; pp 1908-1935.
4. Kienle, R.D.; Kittleson, M.D.: Pulmonary arterial and systemic arterial hypertension. Small Animal Cardiovascular Medicine (M.D. Kittleson; R.D. Kienle, eds.). Mosby, St. Louis, Mo., 1998; pp 433-448.
5. McLaughlin, V.V.; Rich, S.: Cor pulmonale. Heart Disease: A Textbook ofCardiovascular Medicine (E. Braunwald et al., eds.). W.B. Saunders, Philadelphia, Pa., 2001; pp 1936-1954.
6. Klinger, J.R.; Hill, N.S.: Right ventricular dysfunction in chronic obstructive pulmonary disease. Chest99 (3):715-723; 1991.
7. Atkins, C.E.: The role of noncardiac disease in the development and precipitation of heart failure. Vet. Clin. North Am. (Small Anim. Pract.) 21 (5):1035-1080; 1991.
8. Zhao, L. et al.: Sildenafil inhibits hypoxia-induced pulmonary hypertension. Circulation104 (4):424-428; 2001.
9. Michelakis, E. et al.: Oral sildenafil is an effective and specific pulmonary vasodilator in patients with pulmonary arterial hypertension: Comparison with inhaled nitric oxide. Circulation105 (20):2398-2403; 2002.
10. Atkins, C.E.: Cardiac manifestations of systemic and metabolic disease. Textbook of Canine and Feline Cardiology (P. Fox et al., eds.). W.B. Saunders, Philadelphia, Pa., 1999; pp 757-780.
Podcast CE: Canine cardiology: the practical guide to the mitral valve patient
July 19th 2023Learn about the prevalence of myxomatous mitral valve disease, guidelines for staging heart disease, proactive diagnostic workup, the importance of spironolactone and aldosterone blocking, and the benefits of combination therapy for improved outcomes in canine patients
Listen