Three-minute peripheral blood film evaluation: The erythron and thrombon
Evaluating a peripheral blood film validates cell counts performed by hematology analyzers, plus it offers valuable diagnostic information relayed by erythrocytes, platelets, and leukocytes.
Evaluating a peripheral blood film validates cell counts performed by hematology analyzers, plus it offers valuable diagnostic information relayed by erythrocytes, platelets, and leukocytes. The first article in this symposium described how to prepare a peripheral blood film. In this article, we discuss important red blood cell (RBC) and platelet number and morphologic changes. And in the next article, we discuss white blood cell (WBC) alterations.
If a patient's RBC mass is reduced, then the patient is anemic. Once anemia is recognized, the next concern is bone marrow responsiveness: Is the anemia regenerative or nonregenerative? If the RBC bone marrow precursor cells respond with increased production of appropriate magnitude, the anemia is regenerative (responsive). If RBC production is not effectively increased, the anemia is nonregenerative (nonresponsive). Keep in mind that a lag of 48 to 72 hours exists before bone marrow responsiveness is perceived in the peripheral blood, so be careful in defining nonresponsiveness.
Evaluating the thrombon by first assessing platelet numbers is another important part of every complete blood count (CBC). Thrombocytopenia is more common than thrombocytosis and can be of great clinical importance. Platelet counts below 40,000/µl can lead to spontaneous bleeding. Platelet clumping, which can be seen in any species but is particularly problematic in cats, can give a falsely low platelet count. Platelet clumping frequently interferes with results from impedance cell counters because aggregated platelets may be included in the RBC or WBC counts, resulting in artifactually decreased platelet counts.1
Our approach to peripheral blood film evaluation uses a question-based format. This systematic approach allows veterinary practitioners and technicians to maximize important visual information.
EVALUATING THE RBCs
Is there evidence of RBC regeneration? (Is polychromasiaor reticulocytosis present?)
In dogs and cats, polychromasia is the principal feature of RBC regeneration on a blood film prepared with Wright's or modified Wright's stain. Polychromatophils are immature RBCs that stain bluish because they contain RNA (Figure 1). Polychromatophils on blood smears prepared with Wright's or modified Wright's stain roughly correspond to reticulocytes on smears prepared with new methylene blue stain. If regeneration is still questionable after you've evaluated Wright's-stained blood films, perform a reticulocyte count with new methylene blue stain.

1. Polychromatophils (arrows) in a dog (modified Wright's stain; 100X). 2. Aggregate (black arrows) and punctuate (red arrows) reticulocytes in a cat. Note that aggregate reticulocytes contain greater than or equal to five basophilic specks (new methylene blue stain; 100X).
When performing reticulocyte counts in cats, it is important to count aggregate reticulocytes (cells containing diffuse accumulations of reticulum) since they represent recent RBC production in response to relatively severe anemia (Figure 2). In cats, aggregate reticulocytes mature into punctuate reticulocytes within 12 hours, and punctuate reticulocytes mature into RBCs in about 10 days. Consequently, elevated punctuate reticulocytes represent RBC regeneration two weeks earlier, whereas aggregate reticulocytes indicate recent regeneration.2 In dogs and cats, peak reticulocyte counts occur four to eight days after the onset of anemia.
As a general guideline, a reticulocyte count above 60,000/µl in cats (aggregate only) and 80,000/µl in dogs indicates a regenerative anemia.3 Dogs with regenerative anemias have reticulocyte counts that are frequently 100,000 to 300,000/µl (dogs with extremely regenerative anemia can have counts of 500,000/µl or greater), while cats typically demonstrate a less dramatic response when only the aggregate reticulocyte counts are evaluated.
Reference laboratories can provide reticulocyte counts, and in some cases, they will automatically add the reticulocyte count to the CBC at an additional charge if anemia is identified. The newer in-clinic, laser-based hematology analyzers automatically provide absolute reticulocyte counts with each CBC.4
Regenerative anemias can be further classified as either blood loss or hemolytic (decreased RBC lifespan) anemias. Whenever you suspect hemolysis, closely examine RBC morphology to detect spherocytes, acanthocytes, schistocytes, RBC inclusions, and parasites (see below). Figure 3 is a flow chart for classifying anemias in dogs and cats
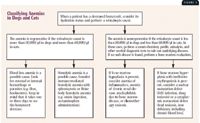
Classifying Anemias in Dogs and Cats.
Are nucleated RBCs present?
Nucleated RBCs, or metarubricytes (Figure 4), are not found in any appreciable numbers in mammalian peripheral blood samples. Most clinicians consider fewer than 4 nucleated RBCs/100 WBCs to be insignificant when the WBC count is within the reference range. Nucleated RBCs may be seen in increased numbers with strongly regenerative anemias, but they should always be less numerous than the polychromatophils or reticulocytes. This finding has been identified as an appropriate nucleated RBC response by some clinicians.

4. A nucleated RBC (arrow) in a dog (modified Wright's stain; 100X). 5. A saline wet preparation of canine blood showing autoagglutination (20X).
An inappropriate nucleated RBC response occurs when more than 5 nucleated RBCs/100 WBCs are present in the absence of polychromasia. Conditions associated with an inappropriate nucleated RBC response include bone marrow stromal damage, extramedullary hematopoiesis, fractures, hyperadrenocorticism, feline leukemia virus infection, chemotherapeutic drug administration, and lead toxicosis, among others.5 Splenic dysfunction (e.g. decreased clearance of circulating nucleated RBCs) is an important cause of an inappropriate release of nucleated RBCs and may occur in patients in which the spleen has been removed and in patients with splenic neoplasms, especially hemangiosarcoma.
Is autoagglutination present?
Agglutination is unorganized three-dimensional clumping of RBCs that must be differentiated from rouleau formation. It is common in dogs with immune-mediated hemolytic anemia. When confirmed, autoagglutination suggests an immune-mediated process such as autoimmune-mediated hemolytic anemia or drug-induced hemolytic anemia (e.g. cephalosporins, penicillin) because RBC surface antibodies cause cell cross-linking and the resulting agglutination.
Rouleau formation is organized linear arrays of RBCs caused by decreased zeta potentials from plasma proteins (e.g. globular proteins, fibrinogen) coating RBCs. This distinctive formation is commonly described as stacks of coins. Unlike agglutination, which is a strong cross-linking between cells, rouleau is a result of a weak binding between cells because of dissimilar ionic charges on the RBC surface. Rouleau formation is a common finding when fibrinogenesis is increased and must be differentiated from agglutination. On routine Wright's-stained or modified-Wright's-stained blood films, marked rouleau formation and agglutination may be difficult to differentiate.
Whenever you suspect agglutination on a blood film, mix a drop of the well-mixed EDTA anticoagulated blood on a new glass slide with two or more drops of isotonic saline solution. Add a cover slip, and view the mixture as an unstained wet preparation. Under these conditions, most rouleaux formations dissipate but autoagglutination typically persists and is recognized as clumped RBCs (Figure 5).
Are poikilocytes present?
Normal canine RBCs are shaped like biconcave disks with prominent central pallor. Feline RBCs have much less apparent central pallor. Abnormally shaped RBCs are called poikilocytes and include artifactual changes (crenation) as well as true abnormalities (e.g. spherocytes, acanthocytes, schistocytes, leptocytes).
Crenation (Figure 6) can be confused with important RBC changes such as acanthocytosis. Crenation is a shrinking artifact most commonly seen when less than optimal amounts of blood are collected into the EDTA tube and the peripheral blood film is not made relatively quickly after the anticoagulation process.4 Some less common causes of crenation include electrolyte disturbances, uremia, and rattlesnake envenomation. A useful differentiating feature for identifying crenation is that crenation typically affects large numbers of cells in a particular area of the slide, whereas true poikilocytosis typically affects relatively lower numbers of cells throughout the peripheral blood film. Crenation can be minimized by preparing blood films immediately after blood samples are collected and properly anticoagulated.
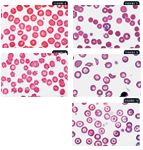
6. Crenation in a dog (modified Wright's stain; 100X). 7. Spherocytes (black arrows) and a polychromatophil (red arrow) in a dog (modified Wright's stain; 100X). 8. Acanthocytes (arrow) in a dog (modified Wright's stain; 100X). 9. Schistocytes (arrows) in a dog (modified Wright's stain; 100X). 10. Leptocytes (arrow) in a dog (modified Wright's stain; 100X).
Spherocytes are spherical RBCs that have lost their normal biconcave shape, resulting in more intense staining than normal RBCs. They have no central zone of pallor, and they appear smaller than normal RBCs (Figure 7). More than four to six spherocytes per 100× field is considered elevated. Spherocytes are commonly seen with many of the immune-mediated hemolytic anemias we encounter in veterinary medicine. Be careful when attempting to identify spherocytes in feline RBCs because feline RBCs are much less biconcave than canine RBCs and, therefore, have much less central pallor.
Acanthocytes are abnormally shaped RBCs having two to 10 blunt, fingerlike surface projections of varying sizes (Figure 8). These morphologic changes are related to abnormal accumulation of lipids within the RBC membrane when there is an abnormal plasma cholesterol:phospholipid ratio. Acanthocytes are seen occasionally in normal animals. Conditions most commonly associated with acanthocyte formation include underlying metabolic diseases or diseases affecting normal lipid metabolism. Nonneoplastic and neoplastic (hemangiosarcoma in particular) diseases involving the liver, spleen, and kidney may have associated acanthocytosis in dogs and, occasionally, in cats.6 Acanthocytosis is most frequently associated with liver disease and splenic hemangiosarcoma.
Schistocytes are RBC fragments formed by mechanical injury (Figure 9). Even in very low numbers (one schistocyte in every three to five 100× objective fields), schistocytes are clinically relevant. Finding even a few schistocytes may help you identify underlying or subclinical disseminated intravascular coagulation (DIC). Microvascular mechanical fragmentation of RBCs associated with diseases such as hemangiosarcoma and dirofilariasis may also result in schistocyte formation.7
A leptocyte (codocyte, target cell) is an RBC with excess cell membrane that forms a shape often referred to as having a Mexican hat appearance (Figure 10). Leptocytes are seen occasionally in normal animals. Increased numbers of leptocytes (> 3/100× oil-immersion field) are expected with polychromasia because polychromatophils have excess membranes compared with normal RBCs. Consequently, leptocytosis is common when reticulocytosis is present, so most reference laboratories do not report this morphologic finding. However, laboratories will report leptocytes when they are seen in the absence of polychromasia since the excess lipid membrane compared with cytoplasmic volume is an abnormality. The two primary mechanisms causing this abnormal morphology are either an upset in the cholesterol:phospholipid ratio in plasma resulting in lipid loading (as might be seen with liver disease and other metabolic disorders) or a decrease in cytoplasmic content compared with normal (as might be seen with iron deficiency typically due to chronic blood loss).8 With iron deficiency, RBC hemoglobin content decreases, and in addition to the leptocytosis, hypochromasia is often observed.
Are RBC inclusions present?
Accurately characterizing various inclusions is an important aspect of blood film evaluation. Inclusions may include Heinz bodies, basophilic stippling, Howell-Jolly bodies, and certain infectious agents.
Heinz bodies are localized accumulations of denatured, oxidized, and precipitated hemoglobin in the RBC. They often affix to the inner RBC membrane and project from the RBC surface. Conditions associated with Heinz body hemolytic anemia include acetaminophen toxicosis in cats and acute onion and zinc toxicosis in dogs. Diabetes mellitus, hyperthyroidism, and lymphosarcoma in cats and the use of oral benzocaine sprays are also associated with increased numbers of Heinz bodies; but anemia is not present in these cases.8 The Heinz bodies observed in cats may be small or not project from the cell surface, making their identification difficult (Figure 11). New methylene blue staining will help confirm the presence of Heinz bodies; the Heinz bodies stain dark blue compared with the rest of the erythrocyte (Figure 12). In contrast to Heinz bodies in dogs, Heinz bodies can be an incidental finding in cats, and their relevance must be determined by evaluating clinical signs and other hemogram parameters.
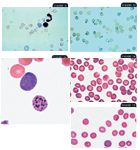
11. Heinz bodies which are noselike RBC projections (arrows) in a cat (modified Wright's stain 100X).12. A blood film from a cat showing Heinz bodies they are easier to see with new methylene blue stain (100X). 13. Basophilic stippling (black arrow) and a polychromatophil (red arrow) in a dog (modified Wright's stain 100X). 14. Babesia canis (arrow) in a dog (modified Wright's stain 100X). 15. Mycoplasma haemocanis (arrow) in a dog (modified Wright's stain 100X).
Basophilic stippling is seen in Romanowsky-stained films as small, dark-blue punctate aggregates of residual ribosomes in RBCs (Figure 13). It has traditionally been associated with lead toxicosis in dogs but may also be associated with highly regenerative anemias in any species. Further diagnostic investigation is important when basophilic stippling is seen in the absence of reticulocytosis.
Howell-Jolly bodies are small nuclear remnants that may be increased with accelerated RBC regeneration. In healthy animals, the low numbers of RBCs with Howell-Jolly bodies released from the bone marrow are quickly removed from circulation primarily through the action of fixed tissue macrophages in the spleen. If Howell-Jolly bodies are easily identified and no reticulocytosis is noted, investigate underlying splenic disease. This inclusion can be an incidental finding in cats because of the open splenic architecture and decreased erythrophagocytic properties inherent to the feline spleen.
Infectious agents including Babesia (Figure 14), Cytauxzoon, and Mycoplasma (previously known as Haemobartonella) species may be identified in RBCs. Evaluation for Mycoplasma species (Figure 15) should be performed on freshly collected blood samples that have not been refrigerated to improve identification.
EVALUATING THE PLATELETS
Are platelet numbers normal, decreased, or increased?
Microscopic validation of platelet counts is an important component of blood film evaluation. Platelet clumping (Figure 16) interferes with accurate enumeration of platelets with all hematology analyzers and with manual counting methods. Clumping occurs in many samples because of platelet activation during the collection process. Increased numbers of large platelets may also result in inaccurate platelet counts when impedance counting methods are used.
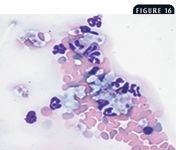
16. A blood film from a cat showing clumped platelets (arrow) (modified Wright's stain; 20X).
On a well-prepared peripheral blood film in which no marked platelet clumping at the feathered edge is present, the average number of platelets observed per 100× oil-immersion monolayer field multiplied by 20,000 provides a good estimate of the number of platelets/µl. As a general rule, you should see a minimum of eight to 10 platelets and a maximum of 35 to 40 platelets per 100× oil-immersion monolayer field of view.9
If the platelet numbers are decreased, can the mechanism be determined?
Thrombocytopenias occur through four basic mechanisms: sequestration, utilization (consumption), destruction, and decreased or ineffective production. While clues as to the underlying mechanism are found in the CBC, in most cases, bone marrow evaluation is needed for complete interpretation.
Sequestration thrombocytopenias are uncommon in veterinary medicine. They are usually the result of hypersplenism and, thus, are characterized by an enlarged spleen on physical or radiographic examination.
Utilization, or consumption, thrombocytopenias are caused by excessive activation of the coagulation cascade. These thrombocytopenias are associated with inflammatory disease and DIC. Utilization thrombocytopenias are usually moderate, with platelet numbers in the 75,000 to 150,000/µl range, but values below 40,000/µl accompanied by petechiae are possible.10 Bone marrow evaluation reveals normal to increased numbers of megakaryocytes.
Destruction thrombocytopenias are immune-mediated thrombocytopenias in which normal circulating platelets are destroyed by circulating antiplatelet antibodies. Destruction thrombocytopenias are often extreme, with peripheral platelet counts well below 50,000/µl. Bone marrow examination is characterized by normal to increased numbers of megakaryocytes.
Decreased or ineffective production is associated with extremely low platelet counts; counts well below 50,000/µl are common. Bone marrow evaluation will vary greatly in most of these cases, although they will share similar end results of deceased effective production by the bone marrow. With decreased production thrombocytopenias, no identifiable to extremely low numbers of megakaryocytes are present. With ineffective production, most bone marrow samples have normal to increased numbers of megakaryocytes, and the production of platelets is ineffective in many cases because of an immune-mediated destructive process directed at an early stage of platelet development or at the megakaryocyte population itself.
If platelet numbers are increased, is the thrombocytosis reactive or neoplastic?
Most thrombocytosis is reactive or neoplastic. Reactive thrombocytosis can occur secondary to exercise, hemorrhage, splenectomy, excitement, fractures, high circulating glucocorticoid concentrations, myelofibrosis, and iron deficiency anemia as well as 24 hours or more after blood loss.11 When the possible causes of reactive thrombocytosis have been ruled out, then the possibility of primary platelet leukemia must be considered. When extremely high platelet counts are seen (> 1,000,000/µl), thrombocytosis due to neoplasia must be strongly considered.
Are enlarged platelets present?
The presence of enlarged platelets (Figure 17), which correlates with increased mean platelet volume (MPV), is supportive of an increased rate of thrombopoiesis in the bone marrow in response to a peripheral demand. This interpretation can be used with most species; however, in cats, enlarged platelets is an equivocal finding.
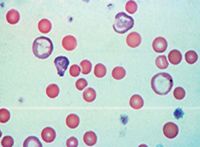
17. A blood film from a dog with immune-mediated hemolytic anemia. Note the enlarged platelets (black arrows). Also note the polychromasia, spherocytosis, and Howell-Jolly body (red arrow) (modified Wright's stain; 100X).
CONCLUSION
Determining if an anemia is regenerative or nonregenerative is critical when developing a list of differential diagnoses. In addition, automated platelet counts should be verified by examining blood films because platelet clumping is common, especially in cats, which results in artifactually decreased platelet counts.
Fred L. Metzger Jr., DVM, DABVP (canine and feline practice)
Metzger Animal Hospital
1044 Benner Pike
State College, PA 16801
Alan Rebar, DVM, PhD, DACVP
Department of Veterinary Pathobiology
School of Veterinary Medicine
Purdue University
West Lafayette, IN 47907
ACKNOWLEDGMENT
The authors gratefully acknowledge the technical assistance and images provided by Dennis DeNicola, DVM, PhD, DACVP.
REFERENCES
1. Rebar A.H. et al.: Laboratory methods in hematology. A Guide to Hematology in Dogs and Cats. Teton New Media, Jackson, Wyo., 2002; pp 3-36.
2. Duncan, J. et al.: Erythrocytes. Veterinary Laboratory Medicine, 3rd Ed. Iowa State University Press, Ames, 1994; pp 3-61.
3. Feldman, B.F. et al.: Reticulocyte response. Schalm's Veterinary Hematology, 5th Ed. Lippincott Williams & Wilkins, Baltimore, Md., 2000; pp 110-116.
4. Rebar A.H. et al.: Erythrocytes. A Guide to Hematology in Dogs and Cats. Teton New Media, Jackson, Wyo., 2002; pp 30-68.
5. Rebar, A.; Metzger, F.: The Veterinary CE Advisor: Interpreting Hemograms in Cats and Dogs. Vet. Med. (suppl.) 96 (12):1-12; 2001.
6. Feldman, B.F. et al.: Classification and laboratory evaluation of anemia. Schalm's Veterinary Hematology, 5th Ed. Lippincott Williams & Wilkins, Baltimore, Md., 2000; pp 140-150.
7. Rebar A.H. et al.: Platelets. A Guide to Hematology in Dogs and Cats. Teton New Media, Jackson, Wyo., 2002; pp 113-134.
8. Thrall, M.A.: Erythrocyte morphology. Veterinary Hematology and Clinical Chemistry. Lippincott Williams & Wilkins, Philadelphia, Pa., 2004; pp 69-82.
9. Duncan, J. et al.: Erythrocytes, leukocytes, hemostasis. Veterinary Laboratory Medicine, 3rd Ed. Iowa State University Press, Ames, 1994; pp 75-93.
10. Harvey, J.W.: Platelets. Atlas of Veterinary Hematology: Blood and Bone Marrow of Domestic Animals. W.B. Saunders, Philadelphia, Pa., 2001; pp 75-80.
11. Feldman, B.F. et al.: Acquired platelet dysfunction. Schalm's Veterinary Hematology, 5th Ed, Lippincott Williams & Wilkins, Baltimore, Md., 2000; pp 496-500.