Allergen-specific immunotherapy for canine atopic dermatitis: Making it work
In this article, I review the efficacy of immunotherapy for treating atopic dermatitis, help you ease your clients' nerves about administering the injections, and show you how to easily adapt the administration protocol based on a patient's responses to the injections.
ALLERGEN-SPECIFIC immunotherapy is the mainstay of therapy for canine atopic dermatitis. But clients may be reluctant to administer the injections at home, and practitioners may find the process of finding the right dose and frequency to achieve an optimal effect frustrating. In this article, I review the efficacy of immunotherapy for treating atopic dermatitis, help you ease your clients' nerves about administering the injections, and show you how to easily adapt the administration protocol based on a patient's responses to the injections.
EFFICACY
Allergen-specific immunotherapy's efficacy in dogs has been demonstrated in a blinded placebo-controlled study that revealed greater than 50% improvement in 59% of allergen-treated dogs and in only 21% of placebo-treated dogs.1 Numerous open studies have demonstrated similar results, with most showing that about 60% of dogs undergoing immunotherapy had good to excellent results. Some prospective studies have shown lower2,3 or higher4,5 (more than 90%) results in dogs that had their allergen-specific immunotherapy protocol altered based on patient response.
One prospective study evaluated allergen-specific immunotherapy in atopic dogs with house dust mite allergy that were treated with house dust mite allergen. This study was the first to monitor symptom, lesion, and medication scores as well as an owner global assessment.6 In 74% of the cases, the baseline symptom and medication scores were significantly reduced at the 12-month recheck, with 63% of cases having greater than 50% reduction. The owner global assessment was improved by more than 50% in 89% of the cases. When combined with other nonsteroidal treatments (antihistamines, fatty acids, and shampooing), allergen-specific immunotherapy was effective in more than 75% of the atopic dogs treated, and the dogs did not need systemic glucocorticoids.7
Allergen-specific immunotherapy is also the only treatment that has been described as curing atopic disease, though controlled studies supporting this statement are lacking.8,9
ADVANTAGES AND DISADVANTAGES
Effective allergen-specific immunotherapy may be more economical than many alternative options, especially in large-breed dogs. On average, a veterinarian pays about $7 for 1 ml of allergen, and most dogs will require 1 to 3 ml a month, with the average case requiring 2 ml of allergen a month. The only other cost is for the 1-ml syringes with needles. Since allergen-specific immunotherapy has not been associated with any organ disease or increased incidence of infections, this therapy does not require monitoring the results of complete blood counts, serum chemistry profiles, or urinalyses. Test costs should be factored in for therapies that require such monitoring. In addition, once maintenance therapy is reached, it is usually less labor-intensive than oral and topical treatments.
The disadvantages of allergen-specific immunotherapy include that it requires subcutaneous injections and that it is not a static therapy. Clinicians must learn how to make changes to optimize its efficacy. Failure to understand allergen-specific immunotherapy and how to modify it leads to frustration, and eventually clinicians abandon this effective and valuable treatment modality. There also may be a long lag time, with some dogs taking nine months to show a beneficial response.1 Published reports do not indicate responses faster than one month, but if glucocorticoids are avoided during the induction phase, it is not uncommon to see responses during the induction phase and the first month of therapy (Figure 1).

Figure 1. Client notes from a partial immunotherapy schedule and the client's assessment of pruritus, with 0 being no itch and 10 being severe itching. The owner assessment was a level 6 when allergen-specific immunotherapy was initiated, and by Day 5 the client assessed a decrease in pruritus by two levels and a greater than 50% reduction in pruritus by Day 9.
SELECTING ALLERGENS FOR IMMUNOTHERAPY
The allergens included in the immunotherapy solution are selected based on several principles, and companies may differ on the number of allergens they will include in a treatment solution. In general, the maximum number will be included as long as the dog reacts to that many allergens and is potentially exposed to them.
Dermatophagoides species allergen is always included if a dog has any allergic reaction to this mite, even if a mild one (pruritus level 2+). After that, the stronger reactions (3 or higher) are initially selected, and then milder (2+) reactions are selected to reach the maximum number allowed in the treatment mix.
Mold allergens are not included unless a dog has strong reactions to them. Pollen allergens are included and are partially selected based on seasonal exacerbations. For example, dogs with clinical signs that are worse in the early spring will be treated for even mild reactions (2+) to trees that are known to pollinate in that season. The allergen company should be able to inform you about plant pollination timing for allergens of interest.
Insect allergens may be present year-round, although they are usually worse in the summer. With insects, it is important to ask the client about possible exposure. Patients may exhibit cross-reactions with cockroach allergen and multiple insect allergens, and dogs known to be exposed (either indoors or outdoors) to many insects such as beetles or silverfish may have cockroach allergen included in their immunotherapy solution. Although clients may think their dogs are not exposed to ants and flies, these allergens are included if allergic dogs spend a lot of time outside. Flea allergen is only included when a dog appears flea allergic even when good flea control is used, when fleas are not found although outdoor exposure is likely, or when a dog's exposure to fleas can't be controlled.
The selected allergens should be those the dog is likely exposed to, though many allergens, especially pollens, are airborne and may travel many miles and persist indoors longer than they are present outdoors. When numerous allergens must be left out, selected allergens from each group (e.g. weeds, trees, grasses, epithelials, insects) can be used. However, in regions in which Bermuda grass grows, Bermuda grass allergens are always included because this type of grass does not stimulate cross-reactions well with other grasses. Some of the weaker-reacting grass allergens may be left out since they more likely share cross-reacting allergens with other grasses. Other components, including pollens, are picked based on which types are known to be more allergenic (cause reactions in a higher percentage of dogs). This information also differs by region and can usually be acquired from the company making the allergen solutions.
VIALS AND PROTOCOLS
Allergen companies will initially supply allergens in two or three vials. Each vial will contain the allergens mixed in a liquid diluent, often referred to as the allergen solution. And each vial of allergen solution will be a particular concentration. The most dilute, lowest concentration allergen solution vials are used initially, and the treatment follows a protocol to gradually build to the highest concentration of allergen solution.

How does allergen-specific immunotherapy work?
The concentration is usually defined as protein nitrogen units (PNU) or weight to volume. In the protocols I use, the concentration of the initial allergen solution (vial 1) is generally 1,000 to 2,000 PNU/ml. The vial that becomes the maintenance vial typically has a total allergen solution concentration of 10,000 to 20,000 PNU/ml, with the largest concentration per allergen usually being 2,000 PNU/ml and infrequently up to 6,000 PNU/ml.
Allergen companies will supply the treatment protocols. The protocol begins with the lowest concentration of allergens and gradually increases the injection volumes and allergen concentration until a maintenance dose is reached. Numerous protocols for allergen-specific immunotherapy are used. All protocols are a starting point, and adjustments may and often should be made based on a pet's response. For dogs, I use two different protocols, one for small dogs (20 lb and under) and one for large dogs (over 20 lb) (Figures 2 & 3).

Figure 2. The hyposensitization schedule for dogs weighing 20 lb or less. The client fills in the date the injections are given and the dog's pruritus score from 0 (none) to 10 (severe pruritus).
TEACHING CLIENTS TO GIVE ALLERGENS AT HOME
Generally, it is easiest to have clients administer the allergen-specific immunotherapy at home with subcutaneous injections (see the client handout "Allergen-specific immunotherapy: 4 easy steps for home administration" ). Some veterinarians and owners are reluctant to do so, but most clients can easily administer the injections, and most dogs will readily tolerate this therapy. The most demanding protocols start with an injection given every other day and end with maintenance injections being given every seven to 30 days. This treatment protocol is certainly less demanding than that needed to treat a diabetic dog or cat.
First, explain the protocol and appropriate vials for each segment of therapy to the client. Once the client understands what the vials are and how the protocol works, teach him or her how to accurately draw up the appropriate amount of antigen solution. Provide 1-ml syringes with 27-ga needles for injecting the solution into a pocket of subcutaneous tissue formed by raising and folding the skin. In my experience and that of many dermatologists, aspiration to assess whether the needle is in a blood vessel is not recommended with this technique and needle size because hitting a vein in the pocket is unlikely and drawing back on the plunger with this size of needle does not readily aspirate blood unless a large vein is penetrated.
Tell clients to use new syringes with each injection; boxes of syringes that are not individually packaged are acceptable and less expensive. Also counsel clients about proper syringe and needle disposal (check your area's medical waste ordinances), preferably into a small sharps container that you send home with them, and instruct them to return the full containers to the clinic for disposal. Be sure to warn clients about avoiding puncturing their own skin. They should be especially cautious if they are known to have any allergies. One client who accidentally punctured his own finger while trying to give an injection to his dog developed anaphylaxis and was hospitalized. He was highly allergic to one of the allergens in the allergen solution.10

Figure 3. A similar schedule to that in Figure 2 but for dogs weighing more than 20 lb. Note the differences in the maximum dose received and injection intervals. At the recheck examination at the end of this schedule, if the dog is doing well, you can try to lengthen the number of days between injections.
Next, demonstrate how to give the injections. Use saline solution if the demonstration is done on the day of an intradermal skin test because if a reaction occurs that day it can be determined that the reaction was from the intradermal test and not from the injection. If a client is picking up the allergen solution after a blood allergy test or at least a day after an intradermal test, then do the demonstration by administering the first dose listed on the immunotherapy protocol so the dog can be observed for any immediate reaction while in the clinic. The client then should practice the injection in front of the instructor with saline solution.
The injections are usually given in the dorsal lateral caudal cervical area, or the nape of the neck. It is helpful to vary the injection locations so that the same site is not repeatedly injected. The injections can be rotated to alternate sides of the neck as well as given slightly more cranial and caudal. Some dermatologists have the client locate the center of the neck and vary the injection sites by visualizing a clock and giving subsequent injections at the various hours rotating consecutively around the clock. If the injection location is not varied, pain or persistent swelling and increased skin thickness may occur, though these problems are rare.
The technique I prefer to teach is to grasp the skin with the thumb and middle finger and slightly lift the skin (Figure 4). Then place the index finger between the thumb and middle finger (Figure 5) to indent the fold of skin being held up so that it forms a Y or V shape (Figures 6 & 7). The needle should be inserted parallel or at up to a 30-degree angle from the plane of the back or neck (Figure 8). Insert the needle rapidly, and then press the plunger. Caution owners about not putting pressure on the plunger as they insert the needle; instead they should wait to put a finger on and press the plunger until the needle is located subcutaneously. To avoid plunger pressure, have clients hold the syringe like a dart and touch the plunger only after inserting the needle through the skin (Figure 9). Also, they need to point directly to the center of the pocket because an angled direction may place the needle through the fold, resulting in the solution being injected outside the skin.

Figure 4. Preparing to grasp the dog's skin with the thumb and middle finger.
Many clients are initially concerned about administering the injections and should practice this technique with a technician or veterinarian present. To alleviate a pet's reluctance to allow the injections at home, the client should draw the solution into the syringe and let the solution come to room temperature before giving the injection as well as train the pet that receiving the injection is good. This training is especially helpful when owners are nervous about giving the injections, as the dogs will often sense this apprehension. Training is accomplished by taking the vial from the refrigerator every day, acting as if the allergen solution is being drawn up, positioning the dog and making a skin fold, touching the fold with a covered needle, and then giving a reward. The client should give a real injection only every other day during the induction and less often after that. Rewards should be given after the real injections as well. Many dogs can be trained to happily anticipate receiving their injections instead of hiding when the refrigerator is opened. For some dogs it is best to only give treats or rewards after the dog has sat still for a practice injection once or even several times a day. They are not given rewards at other times on those days.

Figure 5. Slightly lift the skin, and then place the index finger between the thumb and middle finger.
MONITORING FOR REACTIONS
You must educate clients about what types of reactions they need to watch for. Clients should be able to observe their dogs for the first 30 minutes and preferably one hour after each injection. Any reactions should be noted and communicated to the veterinarian.

Figure 6. Indent the fold of lifted skin so that it forms a Y or V shape.
Life-threatening reactions
Reactions that require contacting the veterinarian immediately include hives, facial swelling, vomiting, diarrhea, weakness, or collapse. These last three problems may indicate anaphylaxis, a potentially life-threatening situation. In one study, anaphylaxis was seen in only one of 185 dogs receiving allergen-specific immunotherapy.11 Usually dogs will exhibit less severe reactions with the injections preceding the development of anaphylaxis. One study reported adverse reactions as early as Day 6 of therapy or as late as Day 142.12
Fatal reactions have been rarely and anecdotally described. I have practiced more than 25 years as a dermatologist in a referral setting, seeing 100% dermatology cases only. In addition, I work with 13 other dermatology specialists at three full-time and six part-time veterinary dermatology practices, and together we have treated thousands of dogs with immunotherapy. In all these clinics, only one dog has had a fatal reaction. I am also a consultant for a commercial laboratory that serves veterinarians in four states and have not learned of other reports of immunotherapy that resulted in anaphylaxis and death.

Figure 7. The V-shaped skin fold in a shorthaired dog.
Reactions that require immediate veterinary attention can occur with any injection, though more severe reactions usually occur in the first few months of therapy and can occur as early as the first injection.
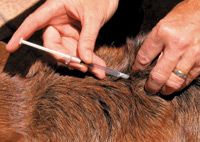
Figure 8. Insert the needle into the center of the V-shaped skin fold parallel or at up to a 30-degree angle from the plane of the back or neck.
Treatment for urticaria is usually limited to hydroxyzine (2.2 mg/kg orally b.i.d. for one or two days). If this is not effective, then prednisone (1 mg/kg orally) is usually effective; if the immunotherapy injections caused the urticaria, then one or two days of prednisone therapy is all that is required.
Angioedema is treated with oral or injectable methylprednisolone (1 mg/kg) and diphenhydramine hydrochloride (2.2 mg/kg) given once.
Anaphylaxis is the most serious complication, and dogs should be treated as soon as possible with intravenous methylprednisolone (1 mg/kg) and diphenhydramine hydrochloride (1 mg/kg not to exceed 40 mg). If a dog presents in severe shock (i.e. has collapsed and has hypotension), then intravenous epinephrine (0.01 mg/kg) should also be given. Some dogs will improve within 15 to 30 minutes even without treatment and may have already recovered while the clients were bringing the dog to the clinic for care. In these cases, treatment may not be required or antihistamines may be given orally.
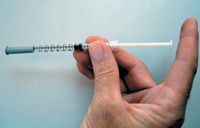
Figure 9. Owners should hold the syringe like a dart and touch the plunger only after inserting the needle subcutaneously.
Non-life-threatening reactions
Evidence of milder allergic reactions that should be reported to the veterinarian before a client gives any more injections are increased itching, listlessness, sleepiness, and anxiousness. Pain or swelling at the injection site is infrequent and minimal if injections are given correctly and animals are trained to accept the injections. If an owner is varying the injection location as instructed, these reactions may be due to inadvertent intradermal injection. Other reactions that may be seen are panting, hyperactivity, increased bowel sounds, changes in urinary habits, and frequent swallowing.
Most reactions occur relatively rapidly after the injections, but changes in activity and pruritus may occur one or two days later. Patterns of pruritus in relation to when injections are given should be monitored, as this is often the basis for making adjustments in the treatment protocol. It is helpful to have clients grade pruritus and note its location. Clients should grade their dogs' pruritus from 0 (no pruritus) to 10 (most severe pruritus for this dog before hyposensitization) before starting the therapy and as frequently as they can during the first few months of therapy. Encourage clients to keep a diary of their observations, which may be helpful in determining allergen adjustments.
ADJUSTING THE PROTOCOL
Immunotherapy protocol adjustments are often needed within the first two to four months, so rechecks should be scheduled. Some dogs require adjustments within the first month, and this can be determined only if the dogs are not receiving glucocorticoids during the induction phase. If a pet reacts during the induction phase, go back to the last dose that produced no reaction, repeat it twice, and then see if you can gradually increase the dose to the amount indicated in the protocol. If the pet still reacts, then the last dose it did not react to is that pet's maximum injection volume and concentration.
There are many ways to try to adjust allergen-specific immunotherapy:
- Change the volume of antigen solution and, therefore, the total amount of protein injected.
- Change the interval between injections so that injections are given more frequently or less frequently.
- Along with giving the original set of antigens, start additional therapy by using a second set of antigens containing either other allergens the pet is allergic to or a combination of some of the original antigens and new antigens.
- Remix or reformulate the antigens to adjust the concentration of antigen (protein level/volume) and the ingredients in each set of antigen.
Most commonly, I change the volume of antigen solution injected or the frequency of the injections. My rule of thumb for injections is to try to reach an average dose of 0.1 ml/day. If a dog is not responding or is responding well, then I will slowly reduce the average dose to 0.05 ml/day. Initially, if the injection interval is between 10 and 20 days, then I will keep the dose at 1 ml (e.g. an average dose of 0.1 to 0.05 ml/day) unless there are reactions correlated with the injections. If injections are given less often than every 10 days, then the dog does not receive the full 1 ml. Dogs that receive injections every five days usually get 0.5 ml or less. If dogs are on a seven-day injection interval, the most that should be given is 0.7 ml. However, occasionally I will give more than this dose but it will be a gradual increase, not a sudden one.
Reasons to adjust allergen-specific immunotherapy after the first four months vary but mainly relate to improving efficacy or decreasing adverse reactions. Animals that have not had any adverse reactions and slight but insignificant improvement after four months are given injections at 10- to 14-day intervals. If they still do not respond by six months, then the volume is lowered to 0.7 ml. If they still do not respond and no pattern is seen, the dosage is changed to 0.5 ml every five to seven days. If there is still no response when those allergens are about gone, consider a new formulation or combination of two formulations.
More commonly, an animal improves after an injection but its clinical signs recur before the next injection is due. In these cases, the frequency of injections is decreased to prevent that increase in clinical signs. Other patients get worse after an injection and then improve, only to start getting worse before the next injection. In these cases, both the volume of antigen and the frequency of injection are decreased. In general, my goal is to find the highest volume that will not cause an increase in clinical signs. Once this goal is accomplished, then the injection frequency is adjusted so that the improvement is maintained the whole time. In some cases in which the frequency cannot be lengthened even at the highest tolerated volume, the frequency is changed to the longest time the pet stays improved and the volume is adjusted, with an average of 0.1 ml/day being the maximum dose.
To transition to a maintenance dose, I keep the dose administered the same, but the number of days between each injection increases. I usually will try to get the maintenance dose to average out to about 0.05 ml/day, and most dogs end up receiving an average of 0.03 ml/day to 0.1 ml/day. Knowing the range of the average daily doses makes it easy to predict the range of what it will cost long-term for the allergen-specific immunotherapy, as that equates closely to 1 to 3 ml a month. The cost of therapy will then be the amount you charge per milliliter, times one to three, plus the cost of syringes based on the frequency of injections per month. When the pet has an excellent response with total control (15% to 20% of cases), then only this cost and one recheck per year is what the owners will spend on controlling their pets' atopic disease.
CONCURRENT THERAPIES
Generally, atopic dogs receiving immunotherapy also should be bathed every one to two weeks and, in flea endemic areas, should receive proper flea control. In a patient that is doing well, fleas will often cause or exacerbate atopic disease as well as induce secondary infections. This may even occur in atopic dogs that are not flea-allergic. Essential fatty acid supplementation or diets high in omega-3 fatty acids may also be beneficial.
Even in cases in which immunotherapy is considered beneficial, an occasional secondary infection may occur and exacerbate pruritus. Clients need to learn about early signs of secondary infections and appropriate intervention with bathing or a recheck examination. You can then prescribe antimicrobial therapy based on cytologic examination or possible culture and sensitivity results if empiric therapy is not effective. This will prevent the secondary problem from aggravating the atopic disease and making the client think the immunotherapy is ineffective or losing efficacy.
In dogs that do not respond to immunotherapy in the first month and in dogs with pruritus that the client cannot tolerate or that the veterinarian feels is excessive based on the severity of skin lesions, concurrent therapies such as antihistamines, glucocorticoids, or cyclosporine may be used. There is no evidence that these concurrent therapies decrease immunotherapy's efficacy. But these adjunctive treatments need to be discontinued every four to six weeks to assess the response to immunotherapy and to determine whether adjustments need to be made.
CONCLUSION
Allergen-specific immunotherapy is a useful tool in treating atopic dermatitis in dogs. Its use is facilitated by client education and training. Although serious adverse reactions are rare, life-threatening reactions may occur and owners need to be educated about them. Minor reactions and poor response are the basis often used for altering protocols and adjusting the therapy. Using adjunctive therapies judiciously and monitoring responses as well as teaching owners to observe their dogs' actions and skin are necessary for optimal management.
The recommendations in this paper are purely mine and are based on more than 25 years of experience managing many thousands of dogs using allergen-specific immunotherapy.
Editors' note: Dr. Griffin is a consultant for Veterinary Allergy Reference Laboratories (VARL). The recommendations in this article are not representative of or endorsed by VARL or any other business or company.
Craig E. Griffin, DVM, DACVD
Animal Dermatology Clinic and Animal Allergy Specialists
5610 Kearny Mesa Road
San Diego, CA 92111
REFERENCES
1. Willemse A, ven den Brom WE, Rijnberk A. Effect of hyposensitization on atopic dermatitis in dogs. J Am Vet Med Assoc 1984;184:1277-1280.
2. Nuttall TJ, Thoday KL, van den Broek AH, et al. Retrospective survey of allergen immunotherapy in canine atopy. Vet Rec 1998;143:139-142.
3. Colombo S, Hill PB, Shaw DJ, et al. Efficacy of low dose immunotherapy in the treatment of canine atopic dermatitis: a prospective, double-blinded, clinical study. Vet Dermatol 2005;16:162-170.
4. Scott DW. Observations on canine atopy. J Am Anim Hosp Assoc 1981;17:91-100.
5. Rosser EJ Jr. Advances in the diagnosis and treatment of atopy. Vet Clin North Am Small Anim Pract 1999;29:1437-1447.
6. Hillier A, Kwochka KW. Allergen-specific immunotherapy in dogs with atopic dermatitis and house dust mite hypersensitivity: use of symptom/medication scores. Vet Dermatol 2002;13:213.
7. Griffin C. Canine atopic disease. In: Current veterinary dermatology: the science and art of therapy. St. Louis, Mo: Mosby-Year Book, 1993;99-120.
8. Power HT. Why do owners discontinue immunotherapy? Vet Dermatol 2000;11(suppl 1):14.
9. Scott DW, Miller WH, Griffin CE. Canine atopic disease. In: Muller and Kirk's small animal dermatology. 6th Ed. Philadelphia, Pa: WB Saunders Co., 2001;574-601.
10. Rosenkrantz WS, Animal Dermatology Clinic, Tustin, Calif: Personal communication, 2004.
11. Zur G, White SD, Ihrke PJ, et al. Canine atopic dermatitis: a retrospective study of 169 cases examined at the University of California, Davis, 1992-1998. Part II. Response to hyposensitization. Vet Dermatol 2002;13:103-111.
12. Rosser EJ. Aqueous hyposensitization in the treatment of canine atopic dermatitis: a retrospective study of 100 cases. In: Kwochka KW, Willemse T, von Tscharner C, eds. Advances in veterinary dermatology. Vol 3. Boston: Butterworth Heinemann 1998;169-176.