The efficacy of an acidified sodium chlorite solution to treat canine Pseudomonas aeruginosa otitis externa
Treating these ears can be frustrating because of changing susceptibility profiles. The organism can become resistant to all available antibiotics or may become susceptible only to expensive or difficult-to-obtain antibiotics.
Canine otitis externa is a common problem, with a reported prevalence of 10% to 20%.1 The most challenging of these cases often involve the gram-negative bacterium Pseudomonas aeruginosa because of its unpredictable antimicrobial sensitivity profile.2 In dogs, P. aeruginosa can be cultured from about 0.4% of normal ears and 20% of otitic ears and is frequently found in chronic, recurrent cases of otitis.3,4 Ears infected with Pseudomonas species are generally painful and often have extensive ulceration of the epithelial lining of the ear canal.4 Treatment typically involves general ear cleansing and may require antimicrobial sensitivity testing to identify the specific topical antibiotic that may be effective. Treating these ears can be frustrating because of changing susceptibility profiles. The organism can become resistant to all available antibiotics or may become susceptible only to expensive or difficult-to-obtain antibiotics. A product that can inactivate isolates with multiple antibiotic resistances would be useful for treating this condition.
We designed a study to evaluate Sanova cleanser (Alcide Corporation) for in vivo efficacy against naturally occurring P. aeruginosa otitis in dogs. Sanova is a USDA-approved antimicrobial solution for use in food processing. The two-part solution consists of a base solution of 2,400 ppm sodium chlorite and an activator solution of 1.2% citric acid. When the liquids are mixed, bioactive chlorine compounds, including chlorine gas and chlorine dioxide, are formed in the solution. These compounds are bactericidal. In unpublished in vitro studies, this cleanser has been shown to effectively destroy isolates of P. aeruginosa and Pseudomonas vulgaris within 15 seconds of contact.5 For this prospective study, we evaluated the effect of this cleanser on objective quantitative culture results and subjective clinical scores.
Materials and methods
Study subjects were client-owned pet dogs with naturally occurring ear disease of at least six weeks' duration that was diagnosed as P. aeruginosa otitis externa upon referral to a specialty veterinary dermatology clinic. The clients signed a consent form, and the patients were enrolled in this study if they met each of the following criteria: a complete otoscopic visualization of the external ear canal and the tympanum revealed abnormalities consistent with an uncomplicated otitis externa; results of cytologic examination of the exudates were consistent with the exclusive presence of Pseudomonas species infection; and culture results were positive for only P. aeruginosa. Visualization with a handheld diagnostic head otoscope and otoscope cone confirmed that the tympanum was intact and that no foreign objects or tumors complicated the infection. If necessary, patients were lightly sedated to allow complete visualization of the external ear canal and tympanum.
We took ear swab samples from the juncture of the horizontal and vertical canals and prepared heat-fixed impression smears on glass slides. We stained the slides with Gram's stain and examined them microscopically for the exclusive presence of large, gram-negative rods consistent with Pseudomonas species.6 We then used sterile swabs to collect samples of the ear exudates from the juncture of the horizontal and vertical canals for bacterial culture and antimicrobial sensitivity testing. The swabs were sent in transport media to a commercial laboratory for bacterial culture and sensitivity testing by using standard disk diffusion techniques and a standard battery of antibiotics. The presence of other organisms identified on cytology or culture resulted in the patient's being excluded from the study. All patients had been treated unsuccessfully with oral antibiotics, ear cleansers, or topical antibiotic and corticosteroid preparations before referral to the specialty dermatology clinic. These treatments were stopped at least 48 hours before enrollment in the study.
After the patients were enrolled in the study, we subjectively scored each ear and performed a quantitative culture. Clinical scoring was based on the amount of exudate present and the condition of the epithelial lining of the ear canal. Exudates were graded as none, scant, moderate, or copious and assigned a score of 0 to 3, respectively. The ear canal epithelium was graded as normal, erythematous, eroded, or ulcerated and also scored from 0 to 3. We added the two parameters to determine the final score.
For the quantitative culture, we instilled 1 ml of sterile saline solution into each affected ear canal, gently massaged the canal for 60 seconds, and then removed 10 µl of the resulting fluid from the vertical ear canal with a sterile, calibrated loop. We then diluted the samples in 1 ml of sterile saline solution. Next, we plated 10 µl of the diluted, agitated sample onto blood agar with another sterile, calibrated loop. Each diluted sample was plated this way in duplicate. We incubated the plates at 98.6 F (37 C) for 24 to 48 hours and then counted the resulting colonies. Plates with colonies too numerous to count were assigned as 4,500 colonies. We chose this number because it is near the limit of the number of colonies that can be accurately counted on a standard agar plate with the naked eye. We performed quantitative cultures one hour before treatment and again just before the initial treatment for comparison and to control for any possibility that the sampling process could distort the number of colonies grown.
Next, one of the investigators performed the initial cleaning with the Sanova cleanser. The sodium chlorite and citric acid solutions were mixed in equal parts just before administration. The cleanser was instilled into each affected ear. Enough cleanser was used to completely fill the patient's ear canal (ranging from 0.5 to 3 ml total volume). The ear was then massaged for 60 seconds and any resulting dislodged debris was wiped out of the ear with cotton balls. Another quantitative culture was performed one hour after the initial treatment.
We then instructed the animal's owners to use the cleanser twice a day on each affected ear for 14 days. The patients' owners performed all subsequent cleanings at home in the same manner as described above. Study animals were to receive no other topical ear preparations, cleansers, systemic antimicrobial medications, or oral glucocorticoids for the duration of the study.
After 14 days of treatment, we reexamined the patients and performed subjective clinical scoring, cytology on impression smears, and quantitative cultures (in duplicate). Cytology and quantitative cultures were repeated as described at least three hours after the last owner-administered treatment.
Statistical analyses
The mean of the two quantitative culture counts from each time interval was calculated. These means were unable to be converted to a normal distribution, so the data were analyzed by using a Wilcoxon signed rank test to determine the P values between the different collection times. Spearman's rank correlation coefficient was used to compare clinical scores and culture counts. All data were analyzed by using Stata 7 (StataCorp). This was an open clinical trial, and no patients were dropped from treatment once enrolled in the study.
The study results
Nineteen dogs with a total of 27 affected ears were enrolled in the study. The nine males and 10 females ranged in age from 1 to 13 years, with a median age of 7 years. All dogs were medium to large in size, and nine breeds were represented.
Sensitivity profiles of the isolates showed that 88.9% had resistance (full or intermediate) to at least one of the tested antibiotics (Table 1). Bacterial isolates were resistant to a median of nine antibiotics. One isolate was resistant to all 20 of the antibiotics for which it was tested.
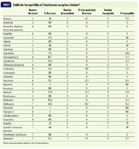
Table 1. Antibiotic Susceptibility of Pseudomonas aeruginosa Isolates
The results of the quantitative culture counts and clinical scores are in Table 2. The counts showed no significant difference (P = 0.600) between the initial samples and those taken just before treatment. However, a significant reduction in the quantitative culture counts was noted between the samples taken just before treatment and those taken one hour after the initial treatment (P < 0.0001). A significant decrease was noted when the counts made one hour before treatment were compared with the counts made after 14 days of treatment (P = 0.0023).

Table 2. Ear Clinical Scores and Quantitative Culture Counts Before and After Treatment
Gram's-stained impression smears from 13 of the 27 ears (48.1%) were free of gram-negative rods after 14 days of twice-a-day treatment. Quantitative cultures showed fewer than five colonies formed in 12 of 24 ears (50%) after 14 days of twice-a-day treatment.
Subjective clinical scores improved significantly after 14 days of treatment (P < 0.0001). Clinical scores did not correlate with quantitative culture counts before treatment (r = 0.34, P = 0.90), but were correlated after treatment (r = 0.51, P = 0.02). Two patients showed a pronounced drying and slight peeling of the external ear canal at the end of the treatment period. However, the owners did not perceive any discomfort for their pets associated with this finding, and both patients returned to normal after treatment was discontinued or the cleanser was used at a less frequent interval.
No patients had treatment discontinued because of adverse reactions, and no owners reported patient discomfort from irritation by the cleanser. Two patients with three otitic ears did not have quantitative cultures performed because the owners failed to return after 14 days. These patients had impression smears and clinical scores performed after 20 and 21 days of twice-a-day treatment. Owner compliance was self-reported as excellent despite many of the dogs being uncooperative during the initial ear cleanings.
Discussion
Sanova cleanser appears to be efficacious in treating dogs with uncomplicated P. aeruginosa otitis externa. Quantitative culture counts, clinical scores, and impression smears showed improvement with twice-a-day treatment. The protocol for quantitative cultures of the ear was used as a way of gathering objective data about the number of colony forming units in the ear exudates. To our knowledge, this type of in vivo quantitative culture count data has not been collected previously. The results of the quantitative culture counts from samples taken from each ear twice before the initial treatment indicate that the sampling process did not significantly alter the number of colonies formed. This was an important consideration and suggests that the significant decrease in culture counts after 14 days of treatment was due to the effect of the Sanova cleanser alone. In the study, 50% of the ears had less than five colonies formed after 14 days of twice-a-day treatment, while 48.1% had negative cytology results. Subjective scores significantly improved with treatment, but did not always correlate with quantitative culture counts. Additional patient data may be required to demonstrate a correlation between the two measures.
All the patients in the study tolerated the ear cleanser well, and no owners expressed dissatisfaction with the cleanser. Although the study included a relatively small number of dogs, it would seem to indicate the product is relatively safe and nonirritating despite its cleaning and disinfecting action.
This study was designed to determine the effect of the cleanser on P. aeruginosa. Although individual isolates from the quantitative cultures were not individually identified, because of the inclusion criteria (Gram's-stained impression smears showing only bacterial morphologies consistent with Pseudomonas species and pure culture isolates of P. aeruginosa documented before quantitative culture collection), it was deemed unlikely that the isolates were other clinically important organisms.7
Alternative treatments or control groups were not used in this study. There are several reasons for this study design. Because of the nature of the condition, a crossover study is impractical because the use of appropriate treatment may result in clinical resolution.6 Untreated controls were not used because spontaneous cure was unlikely in patients that had been unsuccessfully treated with topical or systemic therapies for at least six weeks before enrollment in the study. Additionally, Pseudomonas species are a perpetuating factor of otitis capable of maintaining an inflammatory response,6 and all patients had to be treated for humanitarian reasons. We did not determine whether twice-a-day flushing for 14 days with another product would produce similar results, although a single flush with saline solution before the Sanova treatment did not significantly alter culture counts. This suggests that the significantly reduced culture counts are not due to dilution alone.
Underlying causes of ear disease were not addressed in this study because of the study's short evaluation period. We recognize that successful management of ear disease requires diagnosis and treatment of primary and perpetuating factors.
Additional studies are needed to determine whether a more frequent treatment interval or a prolonged treatment period would result in a higher percentage of cures, as evidenced by clinical signs and bacterial cultures. Direct comparisons to products containing appropriate antibiotics (identified by bacterial culture and antimicrobial sensitivity testing) may help in determining the most efficacious treatment protocol. Additionally, Sanova may be more effective when used in conjunction with antibiotics. Developing a commercially available susceptibility test for Sanova would help to determine its proper place in the treatment of individual cases. However, the results of this study demonstrate that this cleanser is generally helpful in treating uncomplicated otitis externa caused by P. aeruginosa and may be especially appropriate when sensitivity data show that other antimicrobial therapy is unavailable or impractical.
Editor's note: This study was presented orally at the American Academy of Veterinary Dermatology and the American College of Veterinary Dermatology Annual Meeting in Kansas City, Mo., in April 2004. The abstracts from this meeting have been published in Vet Dermatol 2004;15(3):199-206.
ACKNOWLEDGMENTS
The authors acknowledge Alcide Corporation of Redmond, Wash., for its material support in the form of providing Sanova for this study. They also thank Chandra Cristofono, CVT, for her technical assistance.
Tim B. Strauss, DVM, DACVD
Tricia M. McKeever, MS, PhD*
Midwest Veterinary Dermatology
11850 Aberdeen St. NE
Blaine, MN 55449
Patrick J. McKeever, MS, DVM, DACVD
McKeever Dermatology Clinics
7723 Flying Cloud Drive
Eden Prairie, MN 55344
*Current address: Division of Respiratory Medicine, University of Nottingham, University Park, Nottingham, UK NG7 2RD
REFERENCES
1. Griffin CE. Otitis externa and otitis media. In: Griffin CE, Kwochka KW, MacDonald JM, eds. Current veterinary dermatology. Philadelphia, Pa: Mosby-Year Book, 1993;245-262.
2. Logas DB. Diseases of the ear canal. Vet Clin North Am Small Anim Pract 1994;24:905-919.
3. Dickson DB, Lore DN. Bacteriology of the horizontal ear canal of dogs. J Small Anim Pract 1983;24:413-421.
4. August JR. Otitis externa in the dog and cat, Part II: Pathogenesis of the disease, in Proceedings. West Vet Conf 1988;162-168.
5. Collaborative Microbiology Laboratories, Stony Brook, NY: Personal communication, 1994.
6. Kowalski JJ. The microbial environment of the ear canal in health and disease. Vet Clin North Am Small Anim Pract 1988;18:743-754.
7. Rosychuk RAW. Management of otitis externa. Vet Clin North Am Small Anim Pract 1994;24:921-952.