Designing a dosing regimen based on time vs. concentration dependency: a dynamic challenge (Proceedings)
Dosing regimens for antimicrobials exemplify the integration of pharmacokinetics (what the body does to the drug) and pharmacodynamics (what the drug does to the body). For antimicrobial therapy, the "body" is the microbe.
Dosing regimens for antimicrobials exemplify the integration of pharmacokinetics (what the body does to the drug) and pharmacodynamics (what the drug does to the body). For antimicrobial therapy, the "body" is the microbe. Integration is based upon what is needed to achieve the pharmacodynamic response – in this case, the minimum inhibitory concentration of the drug of interest for the infecting microbe – and comparing it with what will be achieved at the chosen dose. For this discussion, we will build a dose around a desired pharmacodynamic index (PDI). If the MIC of the infecting is not known, then the MIC 90 is a reasonable surrogate. The MIC90 is the MIC at or below which 90% of the isolates is an sample population of the organism is inhibited. However, a couple of terms need to be defined and addressed first.
Bactericidal versus bacteriostatic drugs
The term "bactericidal" is so often abused that the distinction from bacteriostatic should be underemphasized. Although it is appropriate for clinicians to reach for a drug that is "cidal" rather than "static", it is not appropriate to assume that the ability of that drug to kill rather than simply inhibit an organisms will enhance therapeutic efficacy. This may be true, but only if concentrations of the drug achieved at the site of infection are sufficient to kill the microbe. The term "bactericidal" is an in vitro definition and is based on killing rates (eg, 99.9% reduction in bacterial inoculum within a 24 hr period of exposure) as well as the proximity of the minimum bactericidal concentration (MBC) of a drug to the MIC. The MBC is determined based on kill curves, or following tube dilution procedures: tubes with no observable growth are inoculated on agar gel. If no organism grows on the agar, the organisms were killed in the test tube. The tube with the lowest concentration of drug that yields no growth on the agar gel contains the MBC of drug. For drugs considered "bactericidal", the MBC is within one tube dilution of the MIC, meaning, the organisms were not simply inhibited, but rather, were killed. For "bacteriostatic" drugs, growth on the agar plate will occur for several tube dilutions above the MIC, indicating that organisms were not killed. However, bacteriostatic drugs are capable of killing (eg, some organisms are exquisitely sensitive to the effects of selected drugs; some "static" drugs are acculumulated to concentrations that are likely to be cidal [eg, macrolides and lincosamides in phagocytes; urine concentration). However, killing concentrations are generally more likely to be achieved for a cidal drugs compared to a static concentration. On the other hand, a "cidal" drug may not kill if concentrations are not sufficient, or if condictions preclude its actions (eg, slow growth in an anerobic environment; combination with growth inhibitors). Thus, cidal effects will occur only if adequate concentrations (ie, MIC/MBC) are achieved at the site. The bactericidal nature of a drug often reflects its mechanism of action. Drugs which target ribosomes (eg, tetracyclines, macrolides, lincosamides, chloramphenical) often simply inhibit the growth of the organism, and, because a much higher drug concentration is necessary to kill the organism, in vitro, the MIC is distant from the MBC. Clinically, host defenses must eradicate the infection following treatment with these drugs unless exceptionally high concentrations (ie, the MBC) of these drugs are achieved in tissues. An exception is made for aminoglycosides, whose ribosomal inhibition is so effective that the organism dies. Drugs which target cell walls (beta lactams including penicllins and cephalosporins; vancomycin), cell membranes (bacitracin, polymixin and colistin), and DNA (enrofloxacin, metronidazole), RNA (rifampin) are defined in vitro as bactericidal. Combinations of static drugs can often result in cidal actions. For example, sulfonamides (which target folic acid synthesis) are static, but when used in combination with diaminopyramidines (eg, trimethoprim), the combination is defined in vitro as cidal. Attaining bactericidal concentrations of an antimicrobial is critical for those infections for which host killing is likely to be impaired. These include but are not limited to infections in immune compromised animals (eg, viral infections [parvovirus, panleukopenia, FIV, FeLV), patients receiving glucocorticoids), or in systems characterized by derangements in local immunity (ie, CNS infection for which an marked inflammatory response can be life threatening; osteomyelitis; peritonitis, bacteremia/sepsis, many chronic infections).
Relationship between MIC, Plasma and tissue Drug Concentrations
The parameters that are most predictive of antimicrobial efficacy and lack of resistance are the ratio of Cmax / MIC, the area under the inhibitory curve (AUC/MIC); and the percent time that PDC are above the MIC [T> MIC]. Based on these relationships, two generally categories of drugs have been described. Exceeding the efficacy targets decreases resistance. Dosing regimens can be designed based on population statistics, using MIC 90 (eg, packaged inserts) as a surrogate indicator of what is needed, or using MIC data from a culture report. The design of the dosing regimen depends upon the drug and its relationship between plasma drug concentrations, the MIC of the infecting organisms and whether or not the drug has a substantial post antibiotic effect (PAE). Postantibiotic Effect: The post antibiotic effect (PAE) describes the continued inhibition of microbial growth after a short exposure of the organisms to the drug. The impact of the PAE on antimicrobial efficacy can be profound, particularly for concentration-dependent drugs. It is the PAE that allows some of these drugs to be administered at long intervals. The PAE may be absent for some organisms or some patients (e.g., some immunocompromised patients). In general, concentration- dependent drugs appear to exhibit longer PAE; further, the duration of the PAE may vary with the magnitude of the peak PDC (ie, longer with higher PDC) and is enhanced by combination antimicrobial therapy. PAEs vary with each drug and each organism.
Time versus Concentration Dependent Drugs
The relationship between MIC and the magnitude and time course of PDC allows drugs to be categorized as to either concentration-dependent (sometimes referred to as dose dependent) or time-dependent; these definitions are supported by primarily by in vitro but also in vivo studies.
Concentration dependent drugs
Best represented by the fluoroquinolones and aminoglycosides, are characterized by efficacy best predicted by the magnitude of plasma drug concentration (Cmax ) compared to the MIC of the infecting organism. For such drugs, the magnitude of the ratio generally should be 10 to 12, but ideally is higher for more difficult infections (eg, Pseudomonas aeruginosa, or infections caused by multiple organisms. The duration that PDC is above the MIC is not as important; in fact, efficacy may be enhanced by a drug-free period (i.e., a long interval between doses). For concentration dependent drugs, a dose that is too low is particularly detrimental. As such, concentration-dependent drugs generally can be administered at longer intervals, ie, once a day. Package inserts can be used to demonstrate the design of a dose for a FQ. Using the marbofloxacn MIC 90 for E. coli (0.06 mcg/ml) or Proteus mirabilus (0.125), the target concentration would be 0.06 X 10 or 0.6 mcg/ml and 1.25 mcg/ml, respectively. A dose of 2.75 mg/kg yields a Cmax of 2.0 mcg/ml, or a ratio of 2.0/0.06 or 33 for E coli and 16 for Proteus. As such, theoretically the low dose would be appropriate for treatment of both. For Staph intermedius, with an MIC 90 of 0.25 mcg/ml, 2.5 mcg/ml is the target. The higher dose of 5.5 mg/kg would be more prudent. For organisms with an MIC of 0.5 mcg/ml, the target of 5 mcg/ml could not be reached at 5.5 mg/kg. However, for the fluorinated quinolones (FQ), efficacy also is predicted in vitro by AUC/MIC: a ratio of < 60 renders the drugs bacteriostatic, whereas > 125 results in (slow) killing but also decreases the risk of resistance and > 250 causing more rapid bacterial killing. Thus, resistance might be less likely to develop for FQ characterized by longer half-lives (or for ENR, by the production of an active metabolite). Twice daily administration of an FQ might be indicated for organisms already characterized by low level resistance (see MPC below); however, the once daily dose should be given twice daily in such situations. The use of a second drug in combination with the FQ might also be considered for isolates whose MIC are sufficiently high that a Cmax/MIC >10 is difficult to achieve. Note that difloxacin might be appropriate at the low dose to treat E coli but not Proteus; for the latter, a plasma drug concentration of 18 mcg/ml would be necessary. This is not achievable at reasonable doses.
Time dependent drugs
In contrast to concentration dependent drugs, efficacy of time-dependent drugs (eg, β-lactams) is enhanced if PDC remain above the MIC for the majority (50 to 70%) of the dosing interval; efficacy is best predicted by percent time that PDC are above the MIC [T> MIC]. For such drugs, simply achieving the MIC (Cmax/MIC =1) is insufficient because PDC (and certainly tissue concentrations) fall below the MIC immediately. With time-dependent drugs, generally a Cmax/MIC of 4 is a good starting point because it assures 2 half-lives will lapse before T=MIC. Two more half-lives can then be added to the dosing interval before the next dose must be given if T>MIC 50%. However, while this sounds like a long time, for amoxicillin and cephalexin, with a half-life of about 1.5 hr, the dosing interval can only be 6 hrs if Cmax/MIC = 4. For example, the MIC 90 for Staph intermedius and amoxicillin-clavulanic acid is < 0.5 mcg/ml. Cmax of 5.5 mcg/ml will be achieved at the labeled dose of amoxicillin-clavulanic. The duration of the dosing interval with this dose depends on the number of half-lives that can lapse as drug concentrations decline to the MIC. In one half-life, plasma drug concentrations will be 2.75 mcg/ml; in two half-lives, 1.35 and in three half-lives, 0.65, which is just above the target. The half-life of amoxicillin is at best 1.5 hrs resulting in 4.5 hrs of T>MIC. The dosing interval can be twice this long. Thus, the next dose should be administered at 9 hrs (8 hr). To reach a 12 hr dosing interval, 3 more hours, or two half-lives are needed. T>MIC is needed for one more half-life; thus, the dose needs to be doubled. Thus, to treat an organism with an MIC of 0.5 mcg/ml with amoxicillin-clavulanic acid, a dose of 13.5 mg/kg every 8 hrs, or 27 mg/kg every 12 hrs must be given. The dose would need to be further modified for drug, microbial and host factors. Staphylococcus intermedius is characterized by a low MIC; if we repeated the process for Staph. aureus, its MIC 90 is 4 mcg/ml. At 13.5 mg/kg, drug concentrations will reach the MIC before one half-life lapses. Even for E. coli, with an MIC90 for amoxicillin-clavulanic acid at 1 mcg/ml, a dose of 26 mg/kg every 8 hrs is the minimum that should be considered. The process can be repeated for cephalexin, with a half-life of 1.3 hr. At 25 mg/kg PO, 15 mcg/ml is achieved. The MIC 90 for Staphylococcus intermedius is 2 mcg/ml; a dose of 25 mg/kg achieves 15 mcg/ml. The amount of time that can lapse can be calculated as follows: the Cmax/MIC (15/2)= 7.5. This equates to essentially 8, or 3 half-lives (2*2*2). Thus, 4 hours can lapse during T>MIC; a dosing interval of 8 hrs is indicated. (To check: 15 mcg/ml > 7.5 > 3.85 > 1.9 mcg/ml = 3 half-lives). For Staphylococcus aureus, the MIC90 is 8 mcg/ml; the Cmax/MIC = 15/8 = essentially 2. One half-life can lapse during T>MIC; the dosing interval can be 2 half-lives, or 3 hours long. Increasing the dose to treat St. aureus at a convenient dosing interval is not practical. For E coli, with an MIC 90 of 16 mcg/ml, not even one half-life can lapse. Cephalexin should not be used to treat E coli. In general, for time dependent drugs, especially if the half-life is short, adding an additional dose is more cost effective than increasing the dose. This is in contrast for time dependent drugs that have a long half-life. For example, once daily dosing may be appropriate for cefpodoxime, depending on the organism. According to the package insert, the MIC 90 for both Staph intermedius and E coli is 0.5 mcg/ml. The Cmax at 10 mg/kg achieves 16 mcg/ml. The number of half-lives that can lapse is 16/0.5 = 32 = 5 half-lives (2X2X2X2X2), or 16 > 8 > 4 > 2 > 1 > 0.5. The half-life of cefpodoxime is 4.5 hrs, thus the dosing interval can be 25 hr X 2, or (theoretically) every 2 days. However, the variability in drug concentrations is marked, and prudence suggests that a 24 hour dosing regimen, as is indicated on the label, is appropriate. For Staph aureus, the MIC 90 is 2 mcg/ml. Three half-lives can lapse during T>MIC; 6 half-lives or essentially a day can lapse before the next dose. However, because this facilitates efficacy, but not necessarily avoids resistance, and because these calculations assume all drug in plasma makes it to the site of infection, a 12 hour dosing interval might be more prudent.
For cefovecin, with a 133 hr half-life (due to protein binding which slowly releases the drug), for each 2X Cmax/MIC, 6 days can lapse (assuming time dependency is valid for periods beyond 24 hrs). However, the Cmax must be based on unbound, not bound drug. The Cmax of unbound drug in dogs approximates 4.0 mcg/ml. The MIC 90 for Staph intermedius is 0.25 mcg/ml; approximately 4 half-lives can lapse during T>MIC; approximately 8 half-lives (40 days) can lapse before the next dose is given. However, if the target organism is Staph aureus, with an MIC of 2 mcg/ml, T>MIC for only one half-life and dosing should occur (if indicated) in one week or less. Constant rate infusion or slow release products might be ideal for time dependent drugs with short-half-lives in the critical patient. Slow release products might be considered for time dependent drugs; however, the dose must be designed to assure that the MIC is achieved for the older slow release products because MIC have changed through the years. Azithromycin is another example of a drug with a very long half-life (72 hr) because of tissue distribution and accumulation. Although the drug can be administered at 2 day intervals. depending on the target MIC, note that the drug may not reach steady-state concentrations for 6 to 14 days. Indeed, care must be taken to remember that the maximum effect of any drug with a long half-life will not be achieved for 3 to 5 half-lives and a loading dose might be indicated for such drugs. Finally, some time dependent drugs have a very long half-life.
Designing a dosing regimen based on MIC from a culture report
Based in the Pseudomonas aeruginosa, the dose for amikacin should be sufficient to achieve Cmax/MIC = 10 or 10 X 16 mcg/ml = 160 mcg/ml. Table 1 indicates that 22 mg/kg achieved 64 mcg/ml in the blood stream. One could calculated the dose needed to achieve 160 mcg/ml based on either a proportion of : (160 mcg/ml)/(64 mcg/ml) = 2.5 X 22 mg/kg = 55 mg/kg; or one could calclulate the dose based on target (160 mcg/ml) X Vd = 37 mg/kg. Either calculation is likely to result in the same conclusion: the drug may not be safet at this high dose, even with once daily dosing, and the addition of a second drug is indicated. For enrofloxacin, the target is 1 mcg/ml * 10 = 10 mcg/ml. At 20 mg/kg, enrofloxacin achieves 4 mcg/ml and its active metabolite ciprofloxacin, achieves 2.9 mcg/ml for a total bioactivity of approximately 7 mcg/ml. Interestingly, despite an "I" designation, enrofloxacin comes closer to achieving the target Cmax/MIC of 10 (7 is achieved). For enrofloxacin, a second 20 mg/kg dose could be added. However, the combination of enrofloxacin and amikacin would be a wiser choice. For the MRSA, note that chloramphenicol, despite an "S" designation, requires an MIC of 8 mcg/ml. The dosing table indicates that 55 mg/kg PO will achieve a Cmax of 10 mcg/ml. Thus, not even one half-life can lapse before T>MIC is reached. A dose of 80 mg/kg would achieve approximately 16 mcg/ml, which would allow one half-live of T>MIC, or a 2 half-life dosing interval. The reported half-life is variable; we will us an average of 4 hours. Thus, if the dog could tolerate it (unlikely), a dose of 80 mg/kg every 8 hours would be indicated. However, this would only result in bacteristatic concentrations. The combination with rifampin would at least increase the changes of therapeutic success.
The previous demonstrations have been based on the assumption that all drug in plasma makes it to the site of infection. However, a variety of host, drug and microbial factors should cause the dosing regimen to be modified even further.
Host Factors
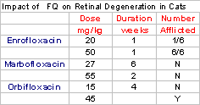
The impact of host response to infection can be profound. Problems contributing to therapeutic failure include immunocompromise (design a dosing regimen that will assure bactericidal concentrations of the chosen drug reach the site of infection), inflammatory response (debride or otherwise appropriate clean/drain accessible infections, select a drug that distributes into tissues well and ideally accumulates in phagocytes and increase the dose appropriately). Interpretation of C&S is based on the assumption that the MIC should be achieved in plasma. Basing MIC interpretation on plasma drug concentrations (PDC) might result in over or under estimation of drug efficacy. For tissues which concentrate the drug (or if the drug can be applied topically), and for drugs which can be concentrated by phagocytes and thus transported to the site of infection, concentrations may markedly exceed PDC, resulting in underestimation of efficacy for several reasons. Much of the data for water soluble drugs (volume of distribution [Vd] generally < 0.3 L/kg) suggests antimicrobial concentrations may be 30% or less of PDC in some tissues, particularly those characterized as sanctuaries, ie, non-fenetrated capillaries. In humans, recommended doses of beta-lactams drugs (water soluble) are increased 5 to 10 fold when treating infections of the central nervous system. Even tissues traditionally considered "well perfused" might be of concern. For example, drugs do not penetrate bronchial secretions well, despite the fact that the lungs are well perfused. Amoxicillin is often used to treat respiratory tract infections. Yet, only 30% of the amoxicillin that is in plasma is distributed to bronchial secretions. Theoretically, one must dose amoxicillin 3X the recommended dose to achieve targeted PDC in bronchial secretions. Most water soluble drugs (beta-lactams and aminoglycosides) reach only 20 to 25% of PDC in bronchial secretions whereas over 50% of lipid soluble drugs reach bronchial secretions. Dosing adjustments also are necessary for those infections that are intracellular or complicated by host response to infection. In the presence of marked inflammation, use of a drug that accumulates in phagocytes (eg FQs, macrolides, lincosamides) is likely to increase distribution of the drug to the site. For fluorinated quinolones and cats, a dose increase should be accompanied by client counseling; of the FQs, marbofloxacin is the last likely to cause retinal degeneration. Cats should be kept inside or way from sunlight after dosing.
Microbial Factors
Microbial resistance is addressed in another manuscript in this same proceedings. Materials released from microbes facilitate invasion, impair cellular phagocytosis, and damage host tissues. Most staphylococci associated with canine pyoderma produce "slime," a material that facilitates bacterial adhesion to cells. Soluble mediators released by organisms (hemolysin, epidermolytic toxin, leukocidin) may damage host tissues or alter host response. Staphylococcal organisms contain protein A, which impairs antibody response, activates complement, and causes chemotaxis. Nocardia stimulates the formation of calcium-containing "sulfur granules" that impair drug penetration to the organisms Pseudomonas and other gram-negative organisms produce a glycocalix, or biofilm, that protects the organism.. Biofilms are microcolonies of pathogenic and host microbes embedded in a polysaccharide matrix ("slime" or "glycocalyx") produced by the bacteria; dental plaque is the prototypic example. Normal microflora of the skin or mucous membranes in the biofilm are lost with shedding of the skin surface or by the excretion of mucus; new cells and mucus are rapidly colonized by biofilm forming bacteria. Translocation of the normal microflora to otherwise sterile tissues (which can be facilitated by the presence of foreign bodies) may lead to acute infections (again, associated with biofilm) and accompanying inflammatory response. Persistent, chronic bacterial infections may reflect biofilm producing bacteria; persistant inflammation associated with immune complexs contributes to clinical signs. Unfortunately, bacteria growing in biofilms more easily resist antimicrobial killing and immune defenses of the host. In addition to debridement or other methods of cleansing should facilitate antimicrobial penetration; dose modification (increase) may be indcated to compensate for debris. Attention to PDC is important not only for efficacy, but also in order to reduce the risk of resistance. For drugs in which resistance emerges as a result of point mutations, dosing regimens should be designed to target the MPC. The mutant prevention concentration (MPC) is defined as the highest MIC identified in a population (= 107 ) infecting the patient (see sister manuscript). The MPC, rather than the MIC, should be the targeted concentration of drug at the site of infection if resistance is to be avoided. Unfortunately, determining the MPC of an isolate cultured from a patient requires culture techniques based on = 107 organisms, which currently is not possible. In one study, the ratio of MPC to MIC for various FQs toward human pathogens ranged from a low of 6 to 10 for E. coli (ATCC 8739) but 23 to 50 and as high as 125 for selected drugs toward Staphylococcus aureus (ATCC 6538).
Drug Factors
In addition to drug characteristics previously addressed (eg, concentration versus time dependent, static versus cidal, drug distribution), pharmaceutical manufacturers have been able to manipulate antimicrobial drugs in a variety of ways such that efficacy and thus bacterial killing is enhanced such that resistance might be reduced. For example, efficacy has been decreased by synthesizing smaller molecules that can penetrate smaller porins (e.g., the extended spectrum penicillins ticarcillin and piperacillin); "protecting" the antibiotic (e.g., with clavulanic acid, which "draws" the attention of the β-lactamase away from the penicillin); modifying the compound so that it is more difficult to destroy (e.g., amikacin, which is a larger and more difficult to reach molecule than gentamicin); and developing lipid-soluble compounds that are more able to achieve effective concentrations at the site of infection (e.g., doxycycline compared with other tetracyclines). However, with each innovative approach to reducing resistance, microbes are able to circumvent the drug in a disconcertingly short time. The use of pro-biotics or pre-biotics to minimize emergence of resistance in the gastrointestinal tract is controversial and requires additional scientific evidence.
Miscellaneous dosing considerations
Generic Augmentin® (human Clavamox®) is now available. However, the ratio of clavulanic acid to amoxicillin varies among the human tablets and solution, but not the small animal versions. The variability reflects an attempt to minimize vomiting. However, it is not clear if the ratio also impacts efficacy. The 400 mg human capsule has the same ratio as the veterinary ratio. Metronidazole benzoate might be compounded for its palatability in cats. However, the benzoate salt weights more than the hydrochloride salt; accordingly, metronidazole benzoate should be dosed at 1.6 times metronidazole hydrochloride (ie, 16 mg/kg versus 10 mg/kg). Ciprofloxacin oral bioavailability in dogs is 40 to 60% and in cats, 0-20%. Although ciprofloxacin is more potent toward Gram negative organisms, the dose should nonetheless be increased two fold compared to enrofloxacin, and 3 fold for Gram positive. Oral ciprofloxacin shold not be used in cats. For other fluoroquinolones in cats, marbofloxacin is among the safest in regards to retinal degeneration.

In conclusion, increasingly, rationale use of antimicrobial therapy should focus on short term therapy with concentrations sufficiently high to kill the infecting microbes, thus assuring that mutation to resistant organisms does not occur. Design of doses are most appropriately based on culture and susceptibility data that indicates the MIC; the closer the MIC is to the breakpoint MIC, the more important increasing doses (of concentration dependent drugs) and shortening the interval of time dependent drugs. Combination therapy is another method whereby the advent of resistance can be minimized. Duration of dosing: Increasingly, in an effort to reduce antimicrobial resistance, the duration of dosing in human medicine is being limited. Durations of 5 days or less are recommended for non-complicated infections. High and/or frequent dosing is intended to result in rapid kill. For slow grow ing organisms, or infections complicated by poor local immunity or prolonged healing, longer durations are indicated. Note that pulse dosing at high, frequent doses intermittently might be preferred to long term dosing at lower doses. The former should be approached such that mutants are killed. In such cases, recurrent infection might be caused by organisms that are not resistant.
