Small animal vaccines and vaccination (Proceedings)
Over the past 3 decades, the number of vaccines licensed for use in dogs and cats has increased significantly. Today, in the United States, the list of companion animal vaccines (proprietary products) numbers about 180.
Over the past 3 decades, the number of vaccines licensed for use in dogs and cats has increased significantly. Today, in the United States, the list of companion animal vaccines (proprietary products) numbers about 180. Because of this unprecedented growth in the vaccine market, scientists, academicians, industry, and practicing veterinarians have met over the past 10 years with the objective of reviewing the most current scientific literature pertaining to infectious diseases and vaccines and to develop vaccination guidelines that could be used by practicing veterinarians in implementing a vaccination protocol. The result of those efforts culminated in the publication of vaccination guidelines. The latest iteration of the canine (2007) and feline (2006) vaccination guidelines can be accessed at no cost through the American Animal Hospital Association and the American Association of Feline Practitioners (AAFP). In 2007, the Vaccine Guidelines Group (VGG) of the World Small Animal Veterinary Association (WSAVA) published the first set of canine and feline vaccine guidelines intended for use by veterinarians in countries outside of North America. WSAVA Vaccination Guidelines have recently been updated (2010). The consistency in recommendations advocated by each of these sets of Guidelines highlights the fact that vaccination is a medical procedure and that the decision to use, or not to use, a particular vaccine in an individual patient is the responsibility of the clinician.
For Veterinary Technician working in private practice, awareness of the recommendations made by the individual practice you work in are important. EVERYONE IN THE PRACTICE SHOULD BE ON THE "SAME PAGE" with respect to vaccine recommendations, especially the CORE vaccines. Awareness of the AAHA and AAFP Vaccine Guidelines are important to technicians who are in position to discuss vaccination decision with clientele. The manuscript that follows provides: 1) a TABULATED summary of canine and feline vaccination guidelines, and 2) a series of FREQUENTLY ASKED QUESTIONS (FAQs) regarding vaccines and vaccination.
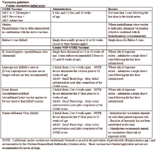
Canine vaccination-adult boosters
• CORE vaccines (distemper+parvovirus+adenovirus-2 and rabies)...single dose every 3 years is recommended.
• Non-CORE vaccines: administer annually where risk of exposure is sustained.
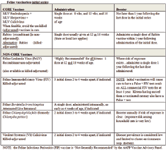
Feline vaccination-adult boosters:
• CORE Vaccines (MLV panleukopenia+herpesvirus+calicivirus): administer a single dose every 3 years following completion of the initial kitten series and the first booster.
• Recombinant (NON-adjuvanted) Rabies: administer a single dose annually in accordance with State or local law.
• Killed (adjuvanted) Rabies: administered in accordance with State or local law.
• Non-CORE Vaccines: (FeLV) recommended annually ONLY if risk is sustained (ie, outdoor cats with reasonable risk of encounter with other cats). The recombinant FeLV vaccine (administered transdermally) is not adjuvanted; all other FeLV vaccines contain adjuvant.
• Other non-core vaccines are seldom administered and should be considered only after assessing and defining a clear risk of exposure. All other non-core vaccines are recommended for annual administration as long as the risk of exposure persists.
References
America Animal Hospital Association (AAHA) Canine Vaccine Guidelines-revised 2007); the full text will be available at www.aahanet.org.
American Association of Feline Practitioners (AAFP) Feline Vaccine Guidelines-2006: available at www.catvets.com.
World Small Animal Veterinary Association's Vaccine Guidelines-2010: available at www.wsava.org.